��Ŀ����
A��B��C��DΪ������Ԫ�أ�B��A���γ�����Һ̬�������ԭ�Ӹ����ȷֱ�Ϊ1��1��2��1���ҷ����е��������ֱ�Ϊ18��10��B��D���γ�һ�ּ�������ˮ�ļ�������X��B��C���γ�һ�ּ�������ˮ����������Y��X������B2A�����еĵ�������ͬ��Y�����еĵ�����Ϊ18��A��B��D�γ����ӻ�����B4A3D2����ˮ��Һ�������ԡ���ش�
��1��Ԫ�ط��ţ�A____________��B____________��C____________��D____________��
��2��д����ѧ����ʽ��C2��X����������Ӧ��_______________________��
��3��B4A3D2�Ļ�ѧʽΪ��_____________________________����ˮ��Һ�����Ե����ӷ���ʽ�ǣ�_________________________________________________��
��1��Ԫ�ط��ţ�A____________��B____________��C____________��D____________��
��2��д����ѧ����ʽ��C2��X����������Ӧ��_______________________��
��3��B4A3D2�Ļ�ѧʽΪ��_____________________________����ˮ��Һ�����Ե����ӷ���ʽ�ǣ�_________________________________________________��
��1��O H Cl N
��2��3Cl2+8NH3====6NH4Cl+N2
(3)NH4NO3 +H2O
+H2O NH3��H2O+H+
NH3��H2O+H+
��2��3Cl2+8NH3====6NH4Cl+N2
(3)NH4NO3
 +H2O
+H2O NH3��H2O+H+
NH3��H2O+H+B��A�γ�����Һ̬��������ԭ�Ӹ����ȷֱ�Ϊ1��1��2��1�����Ʋ�ΪH2O��H2O2���ٽ�ϵ�����Ϊ10��18��֪B��A�ֱ�ΪH��OԪ�ء�B��D�γɼ�������X����DΪ��Ԫ�ء�B��C�γɼ�������ˮ����������Y����Y�����еĵ�����Ϊ18����ôC��Ϊ��Ԫ�ء�

��ϰ��ϵ�д�
 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
�����Ŀ
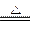 2Ag2O��Ag2O+2HNO3====2AgNO3 +H2O
2Ag2O��Ag2O+2HNO3====2AgNO3 +H2O