��Ŀ����
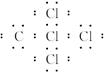
A��B��C��D��E��F���ֶ�����Ԫ�ص�ԭ��������������A��Dͬ�壬B��Cͬ���ڣ�C��F��ͬ���ڵ�����Ԫ�أ�Bԭ�������������Ǵ�����������A��B��Ԫ�صĺ˵����֮��������ǵ�ԭ������������֮�ͣ�E��F��ԭ������֮��Ϊ30������������Ԫ���е�����Ԫ����ɵļס��ҡ����������졢�����ֻ���������ͼ��ʾ��
������������⣺
(1)�����Z�ĵ���ʽ��__________________________��
(2)����1 mol�ҵ�ˮ��Һ�м���MnO2���÷�Ӧ�е���ת�Ƶ����ʵ���Ϊ____________mol��
(3)������������е�ԭ�Ӷ���ͬһֱ���ϣ�ʵ������ȡ���Ļ�ѧ����ʽΪ_______________��
(4)F�ĵ������Ӧ�����ӷ���ʽΪ___________________________��
(5)�����������̼��Ӧ���ɵ����������ˮ��Һ�У�������Ӧ�����ӷ���ʽΪ___________��
| ������ | �� | �� | �� |
| ��Ԫ��ԭ�Ӹ����� | N(A)��N(C)=2��1 | N(A)��N(C)=1��1 | N(B)��N(A)=1��1 |
| ������ | �� | �� | �� |
| ��Ԫ��ԭ�Ӹ����� | N(D)��N(C)=1��1 | N(E)��N(F)=1��3 | N(B)��N(F)=1��4 |
(1)�����Z�ĵ���ʽ��__________________________��
(2)����1 mol�ҵ�ˮ��Һ�м���MnO2���÷�Ӧ�е���ת�Ƶ����ʵ���Ϊ____________mol��
(3)������������е�ԭ�Ӷ���ͬһֱ���ϣ�ʵ������ȡ���Ļ�ѧ����ʽΪ_______________��
(4)F�ĵ������Ӧ�����ӷ���ʽΪ___________________________��
(5)�����������̼��Ӧ���ɵ����������ˮ��Һ�У�������Ӧ�����ӷ���ʽΪ___________��
(1) (2)1
(2)1
(3)CaC2+2H2 Ca(OH)2+ CH��CH��
Ca(OH)2+ CH��CH��
(4)Cl2+H2O====H++Cl-+HClO
(5)3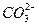 +Al3++3H2O====2Al(OH)3��+3 CO2��
+Al3++3H2O====2Al(OH)3��+3 CO2��
 (2)1
(2)1(3)CaC2+2H2
 Ca(OH)2+ CH��CH��
Ca(OH)2+ CH��CH��(4)Cl2+H2O====H++Cl-+HClO
(5)3
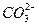 +Al3++3H2O====2Al(OH)3��+3 CO2��
+Al3++3H2O====2Al(OH)3��+3 CO2�����⿼��Ԫ�ؼ��仯����֮��Ľṹ�����ʵĹ�ϵ��
��Bԭ���������������������������2��֪��BΪCԪ�أ�����A��B��C��D��E��F���ֶ�����Ԫ�ص�ԭ��������������A��B��Ԫ�صĺ˵����֮��������ǵ�ԭ������������֮�Ϳ�֪AΪHԪ�أ�A��Dͬ�壬����DΪNaԪ�أ�E��F��ԭ������֮��Ϊ30���ҽ�ϱ����֪��EΪAlԪ�أ�FΪClԪ�ء����Լ�ΪH2O����ΪH2O2����ΪC2H2����ΪNa2O2����ΪAlCl3����ΪCCl4��
��Bԭ���������������������������2��֪��BΪCԪ�أ�����A��B��C��D��E��F���ֶ�����Ԫ�ص�ԭ��������������A��B��Ԫ�صĺ˵����֮��������ǵ�ԭ������������֮�Ϳ�֪AΪHԪ�أ�A��Dͬ�壬����DΪNaԪ�أ�E��F��ԭ������֮��Ϊ30���ҽ�ϱ����֪��EΪAlԪ�أ�FΪClԪ�ء����Լ�ΪH2O����ΪH2O2����ΪC2H2����ΪNa2O2����ΪAlCl3����ΪCCl4��

��ϰ��ϵ�д�
 ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�
�����Ŀ
 ������������������д��Ӧ�������ţ�
������������������д��Ӧ�������ţ�