��Ŀ����
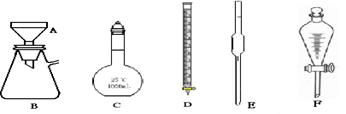
��16�֣���1������д���пհ״���ʵ������Ҫ����500mL0.2mol/L����������Һ��ʵ�鲽������У�
E����ƿ�����������ҡ�ȡ�
F������õ���Һ�����Լ�ƿ�У����ϱ�ǩ����ϴ������ƿ��
��2����16g���������ķ�������ͬ�İ����� g����16g��������ԭ��������ͬ�İ�����
g������ͬ״���У�5.6g������Ӧ���� g����������ɵĻ������������16 g������ռ�е������ȡ�
| A������ƽ�ϳƳ�___________g�����ƹ��壬���������ձ��������������ˮ�ܽ⡣ |
| B���ѵõ�����ҺС�ĵ�����__________ע��________mL������ƿ�С� |
| C������������ˮϴ���ձ��Ͳ�����2��3�Σ�ÿ��ϴ��ҺҲС��ת������ƿ�С� |
| D������������ƿ�м�����ˮ��Һ���̶���������_______________С�ĵμ�����ˮ����Һ��Һ��ײ���̶���ˮƽ���С� |
F������õ���Һ�����Լ�ƿ�У����ϱ�ǩ����ϴ������ƿ��
��2����16g���������ķ�������ͬ�İ����� g����16g��������ԭ��������ͬ�İ�����
g������ͬ״���У�5.6g������Ӧ���� g����������ɵĻ������������16 g������ռ�е������ȡ�
��16�֣�ÿ��2�֣���1��A.14.2 B.������ 500 D. l��2cm ��ͷ�ι�
��2�� 8.5g�� 4.25g�� 5.1g
��2�� 8.5g�� 4.25g�� 5.1g
����һ�����ʵ���Ũ����Һ�����ơ�
��1��500mL0.2mol/L����������Һ�����ʵ����ʵ�����0.5L��0.2mol/L��0.1mol��������0.1mol��142g/mol��14.2g��ת�Ʊ�������ڲ�����������ʱ��Ҫ��ͷ�ιܡ�
��2��16���������ʵ�����16g��32g/mol��0.5mol�����������ʵ���Ҳ��0.5mol����������0.5mol��17g/mol��8.5g��0.5mol��������1.0molԭ�ӣ�������16g��������ԭ��������ͬ�İ�����1mol��4��0.25mol����������0.25mol��17g/mol��4.25g�����ݰ����ӵ����ɿ�֪������������ʵ�����0.5mol�����е�����5.6g��28g/mol��0.2mol�������������ʵ�����0.5mol��0.2mol��0.3mol����������0.3mol��17g/mol��5.1g.
��1��500mL0.2mol/L����������Һ�����ʵ����ʵ�����0.5L��0.2mol/L��0.1mol��������0.1mol��142g/mol��14.2g��ת�Ʊ�������ڲ�����������ʱ��Ҫ��ͷ�ιܡ�
��2��16���������ʵ�����16g��32g/mol��0.5mol�����������ʵ���Ҳ��0.5mol����������0.5mol��17g/mol��8.5g��0.5mol��������1.0molԭ�ӣ�������16g��������ԭ��������ͬ�İ�����1mol��4��0.25mol����������0.25mol��17g/mol��4.25g�����ݰ����ӵ����ɿ�֪������������ʵ�����0.5mol�����е�����5.6g��28g/mol��0.2mol�������������ʵ�����0.5mol��0.2mol��0.3mol����������0.3mol��17g/mol��5.1g.

��ϰ��ϵ�д�
 ���ݼ���ϵ�д�
���ݼ���ϵ�д�
�����Ŀ