��Ŀ����
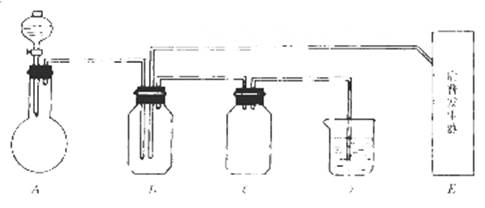
��14�֣���ͼ���й����仯�����ʵ��װ�ã�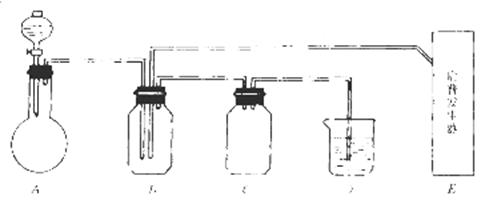
ʵ�����õ���ʵ���Լ���������ѡ��Ũ�����70%�����25%������¿�����������Ʒ�ĩ������������������������Һ������ˮ����̼������Һ��
��ʵ�鿪ʼ���ü���Bƿ�����������ĩ״���ʡ��Իش𣺣��б�ŵı������ţ�
��1��A�з�Һ©��ʢ�ŵ��Լ��� ��
��2��B�з�Ӧ����������ͻ�ԭ��������ʵ������� ��
��3��E�����շ���������ʢ�е������Լ��� ��������Ӧ�����ӷ���ʽ�� ��
��4�����A��E��װ�����巢���ٶ���ͬ������Ҳ��ͬʱ����D�з�Ӧ�����ӷ���ʽ�� ��
��5��D�������� ��C�������� ��
��1���� ��2�� 2:1 ��3���ۢ� FeS+ 2H+ = H2S��+Fe2+
��4��SO2 + 2OH - = SO32-+H2O ��5������β�� ��ȫƿ����
����

��ϰ��ϵ�д�
�����Ŀ