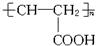��Ŀ����
������A��ʼ������ͼ��ʾ��ת����ϵ�����в��ֲ�������ȥ������֪1 mol F���������Ƶ�Cu��OH��2�ڼ��������³�ַ�Ӧ������2 mol��ɫ������������ͼ���ش����⣺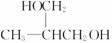
��1��A�к��еĹ����ŵĻ�ѧʽΪ��______________��д���������ʵĽṹ��ʽ��B______________��I______________��
��2��ָ����Ӧ���ͣ�D��G______________��H��I______________��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��
C��F________________________________________________________��
E��H________________________________________________________��
E��M________________________________________________________��
��4����֪һ��̼ԭ�����������ǻ�Ϊ���ȶ��ṹ����C������ͬ���༰��Ŀ�Ĺ����ŵ�ͬ���칹�干��5�֣�������C������CH2��OH��CH��OH��CH2CH3��CH2��OH��CH2CH2CH2OH�⣬�������ֵĽṹ��ʽ______________��______________��______________��
������������֪1 mol F���������Ƶ�Cu��OH��2�ڼ��������³�ַ�Ӧ������2 mol��ɫ������С�⣨4�����Լ�C�ķ���ʽ���ṹ�к��м���������ȷ��C�Ľṹʽ![]() ��H��I��ѧʽ�Ķ��ձȽϿ���ȷ��H��I�������ǼӾ۷�Ӧ��E��H����ȥ��Ӧ��E��M��������Ӧ������M�ķ��ӽṹ�к��а�Ԫ��״�ṹ��������֪E�ĽṹʽΪ
��H��I��ѧʽ�Ķ��ձȽϿ���ȷ��H��I�������ǼӾ۷�Ӧ��E��H����ȥ��Ӧ��E��M��������Ӧ������M�ķ��ӽṹ�к��а�Ԫ��״�ṹ��������֪E�ĽṹʽΪ![]() ��B�ĽṹʽΪ
��B�ĽṹʽΪ![]() ��M�ĽṹʽΪ
��M�ĽṹʽΪ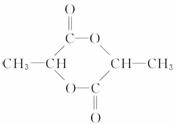
��H�ĽṹʽΪCH2=CHCOOH��DΪNaCl�����B��C����ȷ��A�ڼ��������·���������ˮ���±������ˮ�⣬�����еĹ�������Ȼ����ȷ�ˡ�
�𰸣�
��1����COO������OH����Cl
CH3CH��OH��COONa
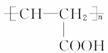
��2�����ֽⷴӦ �Ӿ۷�Ӧ
��3��C��F��
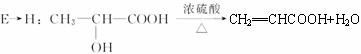
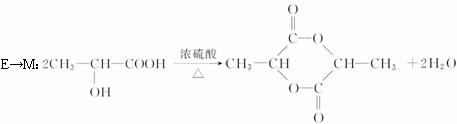
��4��CH2��OH��CH2CH��OH��CH3
CH2��OH��C��OH����CH3��2
CH3CH��OH��CH��OH��CH3

 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д�