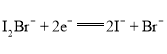ЬтФПФкШн
ЁОЬтФПЁПЛЗМКЯЉЪЧживЊЕФЛЏЙЄдСЯЁЃЦфЪЕбщЪвжЦБИСїГЬШчЯТЃК

ЛиД№ЯТСаЮЪЬтЃК
ЂёЃЎЛЗМКЯЉЕФжЦБИгыЬсДП
ЃЈ1ЃЉдСЯЛЗМКДМжаШєКЌБНЗгдгжЪЃЌМьбщЪдМСЮЊ____________ЃЌЯжЯѓЮЊ__________________ЁЃ
ЃЈ2ЃЉВйзї1ЕФзАжУШчЭМЫљЪОЃЈМгШШКЭМаГжзАжУвбТдШЅЃЉЁЃ

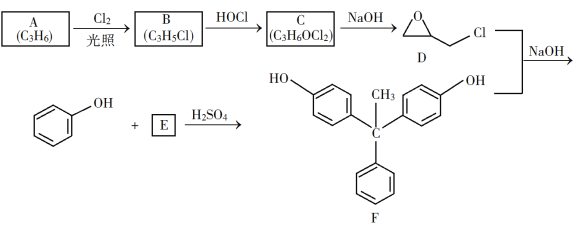
ЂйЩеЦПAжаНјааЕФПЩФцЗДгІЛЏбЇЗНГЬЪНЮЊ________________________ЃЌХЈСђЫсвВПЩзїИУЗДгІЕФДпЛЏМСЃЌбЁдё![]() ЖјВЛгУХЈСђЫсЕФдвђЮЊ________________________ЃЈЬюађКХЃЉЁЃ
ЖјВЛгУХЈСђЫсЕФдвђЮЊ________________________ЃЈЬюађКХЃЉЁЃ
aЃЎХЈСђЫсвзЪЙдСЯЬМЛЏВЂВњЩњ![]()
bЃЎ![]() ЮлШОаЁЁЂПЩбЛЗЪЙгУЃЌЗћКЯТЬЩЋЛЏбЇРэФю
ЮлШОаЁЁЂПЩбЛЗЪЙгУЃЌЗћКЯТЬЩЋЛЏбЇРэФю
cЃЎЭЌЕШЬѕМўЯТЃЌгУ![]() БШХЈСђЫсЕФЦНКтзЊЛЏТЪИп
БШХЈСђЫсЕФЦНКтзЊЛЏТЪИп
ЂквЧЦїBЕФзїгУЮЊ____________ЁЃ
ЃЈ3ЃЉВйзї2гУЕНЕФВЃСЇвЧЦїЪЧ____________ЁЃ
ЃЈ4ЃЉНЋВйзї3ЃЈеєСѓЃЉЕФВНжшВЙЦыЃКАВзАеєСѓзАжУЃЌМгШыД§еєСѓЕФЮяжЪКЭЗаЪЏЃЌ____________ЃЌЦњШЅЧАСѓЗжЃЌЪеМЏ83ЁцЕФСѓЗжЁЃ
ЂђЃЎЛЗМКЯЉКЌСПЕФВтЖЈ
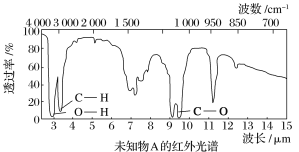
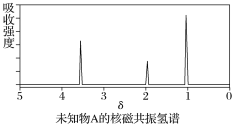
дквЛЖЈЬѕМўЯТЃЌЯђ![]() ЛЗМКЯЉбљЦЗжаМгШыЖЈСПжЦЕУЕФ
ЛЗМКЯЉбљЦЗжаМгШыЖЈСПжЦЕУЕФ![]() ЃЌгыЛЗМКЯЉГфЗжЗДгІКѓЃЌЪЃгрЕФ
ЃЌгыЛЗМКЯЉГфЗжЗДгІКѓЃЌЪЃгрЕФ![]() гызуСП
гызуСП![]() зїгУЩњГЩ
зїгУЩњГЩ![]() ЃЌгУ
ЃЌгУ![]() ЕФ
ЕФ![]() БъзМШмвКЕЮЖЈЃЌжеЕуЪБЯћКФ
БъзМШмвКЕЮЖЈЃЌжеЕуЪБЯћКФ![]() БъзМШмвК
БъзМШмвК![]() ЃЈвдЩЯЪ§ОнОљвбПлГ§ИЩШХвђЫиЃЉЁЃ
ЃЈвдЩЯЪ§ОнОљвбПлГ§ИЩШХвђЫиЃЉЁЃ
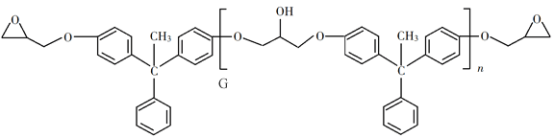
ВтЖЈЙ§ГЬжаЃЌЗЂЩњЕФЗДгІШчЯТЃК
Ђй![]()
Ђк![]()
Ђл![]()
ЃЈ5ЃЉЕЮЖЈЫљгУжИЪОМСЮЊ____________ЁЃбљЦЗжаЛЗМКЯЉЕФжЪСПЗжЪ§ЮЊ____________ЃЈгУзжФИБэЪОЃЉЁЃ
ЃЈ6ЃЉЯТСаЧщПіЛсЕМжТВтЖЈНсЙћЦЋЕЭЕФЪЧ____________ЃЈЬюађКХЃЉЁЃ
aЃЎбљЦЗжаКЌгаБНЗгдгжЪ
bЃЎдкВтЖЈЙ§ГЬжаВПЗжЛЗМКЯЉЛгЗЂ
cЃЎ![]() БъзМШмвКВПЗжБЛбѕЛЏ
БъзМШмвКВПЗжБЛбѕЛЏ
ЁОД№АИЁП![]() ШмвК ШмвКЯдзЯЩЋ
ШмвК ШмвКЯдзЯЩЋ  aЁЂb МѕЩйЛЗМКДМеєГі ЗжвКТЉЖЗЁЂЩеБ ЭЈРфФ§ЫЎЃЌМгШШ ЕэЗлШмвК
aЁЂb МѕЩйЛЗМКДМеєГі ЗжвКТЉЖЗЁЂЩеБ ЭЈРфФ§ЫЎЃЌМгШШ ЕэЗлШмвК  bЁЂc
bЁЂc
ЁОНтЮіЁП
I.ЃЈ1ЃЉМьбщЮяжЪЪБЭЈГЃЪЧРћгУИУЮяжЪЕФЬиЪтаджЪЃЌЛђРћгУВЛЭЌЮяжЪМфЕФаджЪВювьЁЃБНЗгФмгыТШЛЏЬњШмвКЗЂЩњЯдЩЋЗДгІЖјДМВЛФмЃЌПЩвРДЫЩшМЦгУТШЛЏЬњШмвКМьбщБНЗгЕФДцдкЃЛ
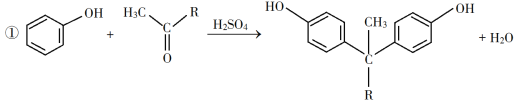
ЃЈ2ЃЉЪщаДФАЩњЧщОГЕФЛЏбЇЗНГЬЪНЪБЃЌвЛЖЈвЊНЋЬтИјЕФЫљгааХЯЂЭкОђГіРДЃЌБШШчЬтИјЕФЗДгІЬѕМўЃЌШчДпЛЏМСЁЂМгШШЕФЮТЖШЁЂДЫЗДгІвбУїШЗжИГіЕФЁАПЩФцЁБЃЛ
ЃЈ3ЃЉДпЛЏМСбЁдёFeCl3ЁЄ6H2OЖјВЛгУХЈСђЫсЕФРэгЩЗжЮіЃЌЯдШЛвЊЭЛГіХЈСђЫсЕФШБЕуЃЌЭЛГіFeCl3ЁЄ6H2OЕФгХЕуЃЛ
ЃЈ4ЃЉдкЗЂЩњзАжУжаМгзАРфФ§ЙмЃЌЯдШЛЪЧЮЊСЫРфФ§ЛиСїЃЌЬсИпдСЯЕФРћгУТЪЁЃ
ЃЈ5ЃЉМЦЫуДЫЮЪЪБОЁПЩФмВЩгУЙиЯЕЪНЗЈЃЌевЕНвбжЊКЭЮДжЊжЎМфЕФжБНгЙиЯЕЁЃ
ЃЈ6ЃЉЮѓВюЗжЮіЪБЃЌРћгУЯрЙиЗДгІЪННЋСПОЁПЩФмУїШЗЛЏЁЃ
I.ЃЈ1ЃЉМьбщБНЗгЕФЪзбЁЪдМСЪЧFeCl3ШмвКЃЌдСЯЛЗМКДМжаШєКЌгаБНЗгЃЌМгШыFeCl3ШмвККѓЃЌШмвКНЋЯдЪОзЯЩЋЃЛ
ЃЈ2ЃЉЂйДгЬтИјЕФжЦБИСїГЬПЩвдПДГіЃЌЛЗМКДМдкFeCl3ЁЄ6H2OЕФзїгУЯТЃЌЗДгІЩњГЩСЫЛЗМКЯЉЃЌЖдБШЛЗМКДМКЭЛЗМКЯЉЕФНсЙЙЃЌПЩжЊЗЂЩњСЫЯћШЅЗДгІЃЌЗДгІЗНГЬЪНЮЊЃК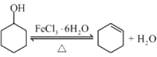 ЃЌзЂвтЩњГЩЕФаЁЗжзгЫЎЮ№ТЉаДЃЌЬтФПвбУїШЗЬсЪОИУЗДгІПЩФцЃЌвЊБъГіПЩФцЗћКХЃЌFeCl3ЁЄ6H2OЪЧЗДгІЬѕМўЃЈДпЛЏМСЃЉБ№ТЉБъЃЛДЫДІгУFeCl3ЁЄ6H2OЖјВЛгУХЈСђЫсЕФдвђЗжЮіжаЃКaЯюКЯРэЃЌвђХЈСђЫсОпгаЧПЭбЫЎадЃЌЭљЭљФмЪЙгаЛњЮяЭбЫЎжСЬПЛЏЃЌИУЙ§ГЬжаЗХГіДѓСПЕФШШЃЌгжПЩвдЪЙЩњГЩЕФЬПгыХЈСђЫсЗЂЩњЗДгІЃКC+2H2SO4(ХЈ)
ЃЌзЂвтЩњГЩЕФаЁЗжзгЫЎЮ№ТЉаДЃЌЬтФПвбУїШЗЬсЪОИУЗДгІПЩФцЃЌвЊБъГіПЩФцЗћКХЃЌFeCl3ЁЄ6H2OЪЧЗДгІЬѕМўЃЈДпЛЏМСЃЉБ№ТЉБъЃЛДЫДІгУFeCl3ЁЄ6H2OЖјВЛгУХЈСђЫсЕФдвђЗжЮіжаЃКaЯюКЯРэЃЌвђХЈСђЫсОпгаЧПЭбЫЎадЃЌЭљЭљФмЪЙгаЛњЮяЭбЫЎжСЬПЛЏЃЌИУЙ§ГЬжаЗХГіДѓСПЕФШШЃЌгжПЩвдЪЙЩњГЩЕФЬПгыХЈСђЫсЗЂЩњЗДгІЃКC+2H2SO4(ХЈ) ![]() CO2Ёќ+SO2Ёќ+2H2OЃЛbЯюКЯРэЃЌгыХЈСђЫсЯрБШЃЌFeCl3ЁЄ6H2OЖдЛЗОГЯрЖдгбКУЃЌЮлШОаЁЃЌОјДѓВПЗжЖМПЩвдЛиЪеВЂбЛЗЪЙгУЃЌИќЗћКЯТЬЩЋЛЏбЇРэФюЃЛcЯюВЛКЯРэЃЌДпЛЏМСВЂВЛФмгАЯьЦНКтзЊЛЏТЪЃЛ
CO2Ёќ+SO2Ёќ+2H2OЃЛbЯюКЯРэЃЌгыХЈСђЫсЯрБШЃЌFeCl3ЁЄ6H2OЖдЛЗОГЯрЖдгбКУЃЌЮлШОаЁЃЌОјДѓВПЗжЖМПЩвдЛиЪеВЂбЛЗЪЙгУЃЌИќЗћКЯТЬЩЋЛЏбЇРэФюЃЛcЯюВЛКЯРэЃЌДпЛЏМСВЂВЛФмгАЯьЦНКтзЊЛЏТЪЃЛ
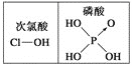
ЂквЧЦїBЮЊЧђаЮРфФ§ЙмЃЌИУвЧЦїЕФзїгУГ§СЫЕМЦјЭтЃЌжївЊзїгУЪЧРфФ§ЛиСїЃЌОЁПЩФмМѕЩйМгШШЪБЗДгІЮяЛЗМКДМЕФеєГіЃЌЬсИпдСЯЛЗМКДМЕФРћгУТЪЃЛ
ЃЈ3ЃЉВйзї2ЪЕЯжСЫЛЅВЛЯрШмЕФСНжжвКЬхЕФЗжРыЃЌгІЪЧЗжвКВйзїЃЌЗжвКВйзїЪБашвЊгУЕНЕФВЃСЇвЧЦїжївЊгаЗжвКТЉЖЗКЭЩеБЃЛ
ЃЈ4ЃЉЬтФПжавбУїШЗЬсЪОСЫВйзї3ЪЧеєСѓВйзїЁЃеєСѓВйзїдкМгШывЉЦЗКѓЃЌвЊЯШЭЈРфФ§ЫЎЃЌдйМгШШЃЛШчЯШМгШШдйЭЈРфФ§ЫЎЃЌБигавЛВПЗжСѓЗжУЛгаМАЪБРфФ§ЃЌдьГЩРЫЗбКЭЮлШОЃЛ
II.ЃЈ5ЃЉвђЕЮЖЈЕФЪЧЕтЕЅжЪЕФШмвКЃЌЫљвдбЁШЁЕэЗлШмвКБШНЯКЯЪЪЃЛИљОнЫљИјЕФЂкЪНКЭЂлЪНЃЌПЩжЊЪЃгрЕФBr2гыЗДгІЯћКФЕФNa2S2O3ЕФЮяжЪЕФСПжЎБШЮЊ1ЃК2ЃЌЫљвдЪЃгр Br2ЕФЮяжЪЕФСПЮЊЃКn(Br2)гр=![]() ЁСcmolЁЄL-1ЁСvmLЁС10-3LЁЄmL-1=
ЁСcmolЁЄL-1ЁСvmLЁС10-3LЁЄmL-1=![]() molЃЌЗДгІЯћКФЕФBr2ЕФЮяжЪЕФСПЮЊЃЈb-
molЃЌЗДгІЯћКФЕФBr2ЕФЮяжЪЕФСПЮЊЃЈb-![]() ЃЉmolЃЌОнЗДгІЂйЪНжаЛЗМКЯЉгыфхЕЅжЪ1ЃК1ЗДгІЃЌПЩжЊЛЗМКЯЉЕФЮяжЪЕФСПвВЮЊЃЈb-
ЃЉmolЃЌОнЗДгІЂйЪНжаЛЗМКЯЉгыфхЕЅжЪ1ЃК1ЗДгІЃЌПЩжЊЛЗМКЯЉЕФЮяжЪЕФСПвВЮЊЃЈb-![]() ЃЉmolЃЌЦфжЪСПЮЊЃЈb-
ЃЉmolЃЌЦфжЪСПЮЊЃЈb-![]() ЃЉЁС82gЃЌЫљвдagбљЦЗжаЛЗМКЯЉЕФжЪСПЗжЪ§ЮЊЃК
ЃЉЁС82gЃЌЫљвдagбљЦЗжаЛЗМКЯЉЕФжЪСПЗжЪ§ЮЊЃК ЁЃ
ЁЃ
ЃЈ6ЃЉaЯюДэЮѓЃЌбљЦЗжаКЌгаБНЗгЃЌЛсЗЂЩњЗДгІЃК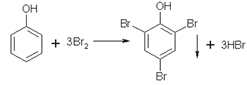 ЃЌУПЗДгІ1molBr2ЃЌЯћКФБНЗгЕФжЪСПЮЊ31.3gЃЛЖјУПЗДгІ1mol Br2ЃЌЯћКФЛЗМКЯЉЕФжЪСПЮЊ82gЃЛЫљвдБНЗгЕФЛьШыЃЌНЋЪЙКФBr2діДѓЃЌДгЖјЪЙЛЗМКЯЉВтЕУНсЙћЦЋДѓЃЛbЯюе§ШЗЃЌВтСПЙ§ГЬжаШчЙћЛЗМКЯЉЛгЗЂЃЌБиШЛЕМжТВтЖЈЛЗМКЯЉЕФНсЙћЦЋЕЭЃЛcЯюе§ШЗЃЌNa2S2O3БъзМШмвКБЛбѕЛЏЃЌБиШЛЕЮЖЈЪБЯћКФЦфЬхЛ§діДѓЃЌМДМЦЫуГіЪЃгрЕФфхЕЅжЪЦЋЖрЃЌЫљвдМЦЫуЕУГігыЛЗМКЯЉЗДгІЕФфхЕЅжЪЕФСПОЭЦЋЕЭЃЌЕМжТзюжеЛЗМКЯЉЕФжЪСПЗжЪ§ЦЋЕЭЁЃ
ЃЌУПЗДгІ1molBr2ЃЌЯћКФБНЗгЕФжЪСПЮЊ31.3gЃЛЖјУПЗДгІ1mol Br2ЃЌЯћКФЛЗМКЯЉЕФжЪСПЮЊ82gЃЛЫљвдБНЗгЕФЛьШыЃЌНЋЪЙКФBr2діДѓЃЌДгЖјЪЙЛЗМКЯЉВтЕУНсЙћЦЋДѓЃЛbЯюе§ШЗЃЌВтСПЙ§ГЬжаШчЙћЛЗМКЯЉЛгЗЂЃЌБиШЛЕМжТВтЖЈЛЗМКЯЉЕФНсЙћЦЋЕЭЃЛcЯюе§ШЗЃЌNa2S2O3БъзМШмвКБЛбѕЛЏЃЌБиШЛЕЮЖЈЪБЯћКФЦфЬхЛ§діДѓЃЌМДМЦЫуГіЪЃгрЕФфхЕЅжЪЦЋЖрЃЌЫљвдМЦЫуЕУГігыЛЗМКЯЉЗДгІЕФфхЕЅжЪЕФСПОЭЦЋЕЭЃЌЕМжТзюжеЛЗМКЯЉЕФжЪСПЗжЪ§ЦЋЕЭЁЃ

 дФЖСПьГЕЯЕСаД№АИ
дФЖСПьГЕЯЕСаД№АИЁОЬтФПЁПЯТСаЮвЙњПЦММГЩЙћЫљЩцМАЮяжЪЕФгІгУжаЃЌЗЂЩњЕФВЛЪЧЛЏбЇБфЛЏЕФЪЧ
|
|
|
|
AЃЎМзДМЕЭЮТЫљжЦЧтЦјгУгкаТФмдДЦћГЕ | BЃЎыЎЁЂыАгУзїЁАШЫдьЬЋбєЁБКЫОлБфШМСЯ | CЃЎЦЋЖўМзыТгУзїЗЂЩфЁАЬьЙЌЖўКХЁБЕФЛ№М§ШМСЯ | DЃЎПЊВЩПЩШМБљЃЌНЋЦфзїЮЊФмдДЪЙгУ |
A. A B. B C. C D. D