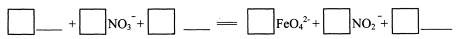��Ŀ����
(14�֣����Ȼ����ı��渺������������������Ĺ�����ܣ������ڹ��������ˮ�е��л���Ⱦ�ij�������������������Ʊ����ֹ��������ͬʱ��ȡ���;�ˮ���������ơ�

(1)д���ٷ�Ӧ�����ӷ���ʽ______________
(2) ���˵õ��Ȼ�����������ϴ�Ӹɾ����ٽ��й��ա������Ȼ����������Ƿ�ϴ���IJ���������____________________________
(3) ��Ӧ�ڵ����ӷ���ʽ��

(4) ��21.60g�������ߵõ���25.15g���ղ������������������ӵ�����������_______����������������λС����
��1��Fe3+ + Ag + Cl- ===AgCl+ Fe2+��3�֣�
��2��ȡ�������ϴ��Һ���μ�KSCN��Һ�������Cl-�����������𰸾����֣�����3�֣�
��3��1 Fe2O3 + 3 NO3- + 4OH- === 2 FeO42- + 3 NO2- + 2H2O ��4�֣�
��4��43�� ��0.43��4�֣�
����������1���ӹ�������ͼ�п��Է�������Ӧ��ΪFe3+����Ag�������ӷ���ʽΪ
Fe3+ + Ag + Cl- ===AgCl + Fe2+
��2����������Ƿ�ϴ���ı�־���ؼ����ҳ����ܸ��ŵĿ��������ӡ�������Լ������һ��ϴ��Һ������Fe3+��Cl-��
��3�������漰������ԭ��Ӧȱ����ƽ����������ͼ�ҳ���ȱ�����ʣ��ٽ�����ƽ���ɡ�
��4�������ò������ҳ����ӵ�����Ϊ��Ԫ�ص�������������Ԫ�ص��������AgCl������������������������������ӵ�����������