��Ŀ����
����ѪҺ�ڵ�Ѫ�쵰��(Hb)����O2�����������Ѫ�쵰�ף�HbO2������˾�������������CO������з�����Ӧ��CO+HbO2 O2+HbCO����������ȱ����ijʵ����ģ���о��������̡�
O2+HbCO����������ȱ����ijʵ����ģ���о��������̡�
��ͼ��ʾHbO2Ũ����ʱ��ı仯������I��ʾ��Ѫ����ͨ��������ı仯�����ߢ��ʾ�ڵ�4sͨ��CO��O2�Ļ������ʹ����ʼŨ�ȷֱ�Ϊ1.0��10-4 mol��L-1��9.9��10-4 mol��L-1��ı仯��
 O2+HbCO����������ȱ����ijʵ����ģ���о��������̡�
O2+HbCO����������ȱ����ijʵ����ģ���о��������̡���ͼ��ʾHbO2Ũ����ʱ��ı仯������I��ʾ��Ѫ����ͨ��������ı仯�����ߢ��ʾ�ڵ�4sͨ��CO��O2�Ļ������ʹ����ʼŨ�ȷֱ�Ϊ1.0��10-4 mol��L-1��9.9��10-4 mol��L-1��ı仯��
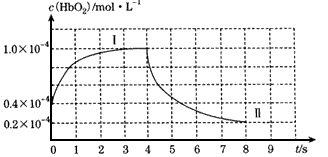
(1)0��3 s����HbO2��ʾ��ƽ����Ӧ����Ϊ___________����9 sʱHbCO��Ũ��Ϊ __________���������ߢ���㷴ӦCO+HbO2 O2+HbCO��ƽ�ⳣ����ֵΪ____________��
O2+HbCO��ƽ�ⳣ����ֵΪ____________��
(2) HbCO��Ũ�ȳ���HbO2Ũ�ȵ�1�����ͻ����ض�һ����̼�ж���֢״�������й�һ����̼�ж���˵���������________��
A�����������COԽ�࣬��Ѫ�쵰��ϵ�O2Խ��
B��������CO��O2�ı�Ϊ1��10ʱ�����ᷢ���ض�һ����̼�ж�
C���������һ����̼�ж�ʱ����ʱ����ͨ�������ڻ����ж����
D��ʹ��ú¯������ȡů���ܻ����һ����̼�ж�
(3)�����˷����ѹ����������һ����̼�ж��ij��÷��������û�ѧ��Ӧԭ������������ԭ��_________��
(4)Ѫ�쵰��Я�����ܺ��京�еĶ���������Fe2+��ʾ����ϵ���У���������������������Ҳ�ᵼ����֯ȱ�����ж���д���������������䷴Ӧ�����ӷ���ʽ______________��
(1)2��10-5 mol��L-1��S-1 ��8��10-5 mol�� L-1 ��214
(2)B
(3)��������Ũ�ȣ�ʹƽ�����淴Ӧ�����ƶ���ʹHbO2Ũ������HbCOŨ�ȼ�С��
(4)2H++NO2-+Fe2+=Fe3+ +NO+H2O
(2)B
(3)��������Ũ�ȣ�ʹƽ�����淴Ӧ�����ƶ���ʹHbO2Ũ������HbCOŨ�ȼ�С��
(4)2H++NO2-+Fe2+=Fe3+ +NO+H2O

��ϰ��ϵ�д�
�����Ŀ
����ѪҺ�ڵ�Ѫ�쵰�ף�Hb������O2�������HbO2����˾�������������CO������з�����Ӧ��CO+HbO2?O2+HbCO��37��ʱ���÷�Ӧ��ƽ�ⳣ��K=220��HbCO��Ũ�ȴﵽHbO2Ũ�ȵ�0.02������ʹ���������𣮾ݴˣ����н��۴�����ǣ�������
| A��CO��HbO2��Ӧ��ƽ�ⳣ��K=[O2]?[HbCO]/[CO]?[HbO2] | B�����������COԽ�࣬��Ѫ�쵰��ϵ�O2Խ�� | C���������CO��O2Ũ��֮�ȵ���0.001ʱ���˵����������� | D����CO�ж����˷����ѹ�����нⶾ��Ŀ���ǣ�ʹƽ�ⳣ����С���������ƶ� |

 O2+HbCO
��37 ��ʱ���÷�Ӧ��ƽ�ⳣ��K=220 ��HbCO��Ũ�ȴﵽHbO2Ũ�ȵ�0.02������ʹ���������𡣾ݴˣ����н��۴������
O2+HbCO
��37 ��ʱ���÷�Ӧ��ƽ�ⳣ��K=220 ��HbCO��Ũ�ȴﵽHbO2Ũ�ȵ�0.02������ʹ���������𡣾ݴˣ����н��۴������
 B.���������COԽ�࣬��Ѫ�쵰��ϵ�O2Խ��
B.���������COԽ�࣬��Ѫ�쵰��ϵ�O2Խ��