��Ŀ����
I��ijѧ����0.2000 mol��L-1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ������ɷ�Ϊ���¼�����
��������ˮϴ�Ӽ�ʽ�ζ��ܣ���ע��NaOH��Һ����0���̶�������
�ڹ̶��õζ��ܲ�ʹ�ζ��ܼ������Һ��
�۵���Һ������0����0���̶������£������¶���
����ȡ20.00mL����Һע����ϴ������ƿ�У�������1��2�η�̪��Һ
�ݵ���һ�α�Һ����Һ��ɫ����ɫ��Ϊ��ɫ����ֹͣ�ζ�����¼Һ�����
��ش�
��1�����ϲ����д�����ǣ����ţ� ��
��2���ñ�NaOH��Һ�ζ�ʱ��Ӧ����NaOH��Һע��_________�С�����ͼ��ѡ��ס����ҡ���

��3�����в���������ʵ����ƫ����ǣ�_________�����ţ�
A����ʽ�ζ���δ��ϴ
B���ζ�ǰ���ζ��ܼ��������ݣ��ζ���������
C����ƿ��������ˮϴ�Ӻ�δ�ô���Һ��ϴ
D���ζ�����ʱ���ӵζ��ܣ�����¼����
E.�ζ���������һ�α�Һ�ɽ�����ƿ
��4���ζ�ʱ�����ֿ��Ƶζ��ܣ�����ҡ����ƿ���� ��ע�� ��
��ע�� ��
II.�����к͵ζ���ԭ�����ڹ�ҵ�����л����Խ���������ԭ�ζ��ⶨ���ʺ�����
�Ʊ�����TiO2�ķ���֮һ��TiCl4ˮ������TiO2��xH2O�������ˡ�ˮϴ��ȥ���е�Cl-���ٺ�ɡ����ճ�ȥˮ�ֵõ���״TiO2��
���ִ����������ⶨTiO2���ӵĴ�С����������ԭ�ζ����ⶨTiO2������������һ�������£���TiO2�ܽⲢ��ԭΪTi3+������KSCN��Һ��ָʾ������NH4Fe��SO4��2����Һ�ζ�Ti3+��ȫ������Ti4+��
��ش��������⣺
��5����ȥTiCl4���е�����A3+��ѡ�õ���pH�ķ���ʹ��ת��ΪA��OH��3������ȥ����֪Al��OH��3��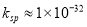 �����뽫Al3+��ȫ�����������������ӵij�����������Һ��pHһ������С��__________����
�����뽫Al3+��ȫ�����������������ӵij�����������Һ��pHһ������С��__________����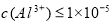 ʱ��������Ϊ��ȫ������
ʱ��������Ϊ��ȫ������
��6��TiCl4ˮ������TiO2��xH2O�Ļ�ѧ����ʽΪ__________________________��
��7���ζ��յ��������___________________________��
��8���ζ�����ʱ����ȡTiO2��Ħ������ΪM g��mol-1������w g������c mol��L-1NH4Fe��SO4��2����ҺV mL����TiO2������������ʽΪ____________________��
 Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д� ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д� ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д� �Ƹ�360�ȶ����ܾ�ϵ�д�
�Ƹ�360�ȶ����ܾ�ϵ�д�

 ������ʯ��ˮ B������������Һ
������ʯ��ˮ B������������Һ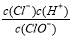 ��С
��С