��Ŀ����
��֪�Ҷ���(HOOC��COOH)�׳Ʋ��ᣬ������ˮ���������������ֽ⡣���β���ƺͲ�����ƾ�Ϊ��ɫ�����ij����С��Ϊ��̽���Ҷ��������ǿ�����Ҷ������ȷֽ������������ʵ�顣
(1)��pH��ֽ�ⶨ�����ʵ���Ũ�ȵ����ᡢ�Ҷ��ᡢ������Һ��pH�������Һ��pH�ֱ�Ϊa��b��c����a��b��c��
�ٲⶨ������ҺpH�ķ�����________________________________________________��
�������������������ǿ������˳����__________________________________________��
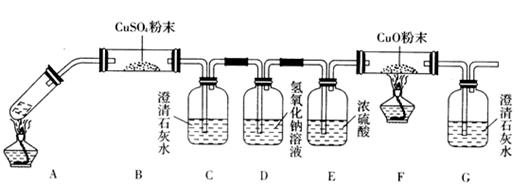
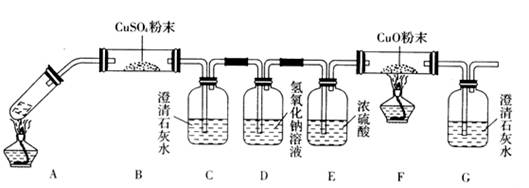
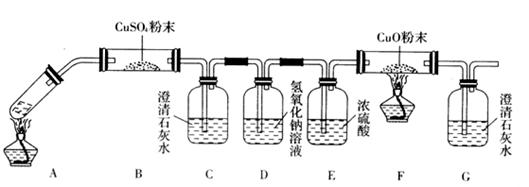
(2)��һ�������Ҷ�������Թ��У�����ͼװ�ý���ʵ�顣

��ּ���һ��ʱ�������װ����U�ι����������Һ�壬��װ���г���ʯ��ˮ����ǡ�����Ӧ��ɺ�����װ����U�ι������������ˮ����ͭ����ˮ����ͭ������
�ٸ�������ʵ����ʵ���Ʋ��Ҷ���ֽ������һ����__________________________________��
����װ�õ�������________________________________________________________��
(3)���ݶ�װ���Ʋ⣬�Ҷ���ֽ�IJ����л�������___________________________________��
(1)���ýྻ�IJ�����պȡ���������Ե���һ��pH��ֽ�ϣ�����ֽ��ʾ����ɫ�����ɫ���Ƚϣ���ȷ����pH
������Ҷ������(��H2SO4��![]() ��CH3COOH)
��CH3COOH)
(2)�ٶ�����̼��ˮ(��CO2��H2O)
�������Ҷ���ֽ����̬������������Ҷ��ᣬ�ų��������Ҷ������ֽ���������̼ȷ���ĸ���
(3)һ����̼(��CO)


 OC�DCOOH���ɼ�дΪH2C2O4)�׳Ʋ��ᣬ������ˮ�����ڶ�Ԫ��ǿ��(Ϊ�������)��������ǿ��̼�ᣬ���۵�Ϊ101.5�棬��157��������ijУ�о���ѧϰС��Ϊ̽������IJ��ֻ�ѧ���ʣ�����������ʵ�飺
OC�DCOOH���ɼ�дΪH2C2O4)�׳Ʋ��ᣬ������ˮ�����ڶ�Ԫ��ǿ��(Ϊ�������)��������ǿ��̼�ᣬ���۵�Ϊ101.5�棬��157��������ijУ�о���ѧϰС��Ϊ̽������IJ��ֻ�ѧ���ʣ�����������ʵ�飺 HCO3��Һ���Թ��м��������Ҷ�����Һ���۲쵽����ɫ���ݲ������÷�Ӧ�����ӷ���ʽΪ_______
HCO3��Һ���Թ��м��������Ҷ�����Һ���۲쵽����ɫ���ݲ������÷�Ӧ�����ӷ���ʽΪ_______ __________________________________��
__________________________________��