��Ŀ����
��13�֣�ij��ȤС��Ϊ��֤�ճ������õĻ��ͷ�Ϻ���KClO3��MnO2��S�����������ʵ������ͼ��
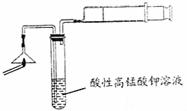
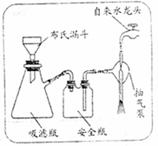
��ش��������⣺
��1��Ϊ��֤����A������ͼ��ʾ����ʵ�飺���ܹ۲쵽 ��������֤�����ͷ�Ϻ���SԪ�ء�
��2��д��������з�����Ӧ�Ļ�ѧ����ʽ ��
��3������ڵ�ʵ�����װ��������ͼ��ʾ���ò����������� ��
��4��Ҫ֤�����ͷ�к���ClԪ�صĺ���ʵ�鲽���� ��
��5����ѧ�����������ͷ��KClO3��һ��ʵ�鷽����
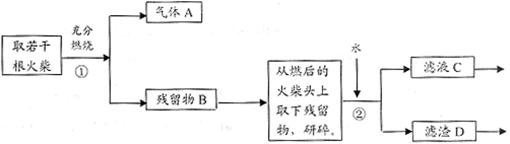
�йص����ӷ�Ӧ����ʽΪ ������������������г��ְ�ɫ���������ܳ��˵�����ͷ��KClO3�Ĵ��ڣ��������� ����6����С��²�����D��˫��ˮ�ֽ������������ʻ����һ����Ӱ�죬��Ʋ�����������5��ʵ�顣
���ϱ���֪��ʵ��ٺ͢���֤���¶�Խ�ߣ���ѧ��Ӧ����Խ�죬ʵ�� ��
֤������D������Խ��Ӧ����Խ�졣



��ش��������⣺
��1��Ϊ��֤����A������ͼ��ʾ����ʵ�飺���ܹ۲쵽 ��������֤�����ͷ�Ϻ���SԪ�ء�
��2��д��������з�����Ӧ�Ļ�ѧ����ʽ ��
��3������ڵ�ʵ�����װ��������ͼ��ʾ���ò����������� ��
��4��Ҫ֤�����ͷ�к���ClԪ�صĺ���ʵ�鲽���� ��
��5����ѧ�����������ͷ��KClO3��һ��ʵ�鷽����

|
| ʵ����� | H2O2��Һ��������% | H2O2��Һ����/���� | ����D����/�� | ��Ӧ�¶�/�� | �ռ��������/���� | ����ʱ��/�� |
| �� | 30 | 5 | 0 | 85 | 2 | 3.8 |
| �� | 15 | 2 | 0.1 | 20 | 2 | 2.8 |
| �� | 15 | 2 | 0.2 | 20 | 2 | 2.2 |
| �� | 5 | 2 | 0.1 | 20 | 2 | 7.4 |
| �� | 30 | 5 | 0 | 55 | 2 | 10.5 |
֤������D������Խ��Ӧ����Խ�졣
��13�֣�
��1��KMnO4��Һ���Ϻ�ɫ����ɫ��1�֣�
 ��2��2KClO3
��2��2KClO3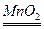 2KCl+3O2����1�֣� S+O2
2KCl+3O2����1�֣� S+O2 SO2 ��1�֣�
SO2 ��1�֣�
��3����ѹ���ˣ�����ˣ���2�֣�
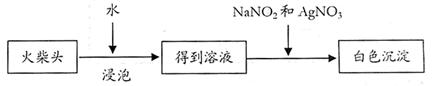
��4��ȡ��ҺC������HNO3��AgNO3��Һ�����۲쵽��ɫ��������������֤�����ͷ�к�����Ԫ�ء���2�֣�
��5��ClO3¯+3NO2¯+Ag+=AgCl��+3NO3¯��2�֣�
AgNO2��AgCl��Ϊ������ˮ�İ�ɫ��������2�֣�
��6���ںۣ͢�2�֣�
��1��KMnO4��Һ���Ϻ�ɫ����ɫ��1�֣�
 ��2��2KClO3
��2��2KClO3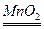 2KCl+3O2����1�֣� S+O2
2KCl+3O2����1�֣� S+O2 SO2 ��1�֣�
SO2 ��1�֣���3����ѹ���ˣ�����ˣ���2�֣�
��4��ȡ��ҺC������HNO3��AgNO3��Һ�����۲쵽��ɫ��������������֤�����ͷ�к�����Ԫ�ء���2�֣�
��5��ClO3¯+3NO2¯+Ag+=AgCl��+3NO3¯��2�֣�
AgNO2��AgCl��Ϊ������ˮ�İ�ɫ��������2�֣�
��6���ںۣ͢�2�֣�
��

��ϰ��ϵ�д�
�����Ŀ