��Ŀ����
20�� ��������͵����������dz��õĻ���ԭ�ϣ���Ҳ�Ǵ�������Ҫ��Ⱦ��ۺ���������Ⱦ�ǻ�����ѧ��ǰ����Ҫ�о�����֮һ��
��������͵����������dz��õĻ���ԭ�ϣ���Ҳ�Ǵ�������Ҫ��Ⱦ��ۺ���������Ⱦ�ǻ�����ѧ��ǰ����Ҫ�о�����֮һ����1�����������У�SO2����������SO3��2SO2��g��+O2��g��$?_{��}^{����}$2SO3��g��
ij�¶��£�SO2��ƽ��ת���ʣ�a������ϵ��ѹǿ��p���Ĺ�ϵ��ͼ��ʾ������ͼʾ�ش��������⣺
�ٽ�2.0mol SO2��1.0mol O2����10L�ܱ������У���Ӧ��ƽ�����ϵ��ѹǿΪ0.10MPa���÷�Ӧ��ƽ�ⳣ������800��
�������������м���1.0mol SO2��0.5mol O2��amol SO3�������¶Ȳ��䷴Ӧ��ƽ�����ϵ��ѹǿҲΪ0.10MPa����a=1.0mol��SO2��ƽ��ת����Ϊ60%��
��ƽ��״̬��A�䵽Bʱ��ƽ�ⳣ��K��A��=K��B�������������������=������
��2����CH4����ԭNOx�������������������Ⱦ�����磺
CH4��g��+4NO2��g���T4NO��g��+C02��g��+2H20��g����H=-574kJ•mol-1
CH4��g��+4NO��g���T2N2��g��+CO2��g��+2H20��g����H=-1160kJ•mol-1
���ñ�״����4.48L CH4��ԭNO2��N2���������У��ų�������Ϊ173.4kJ��
���� ��1���ٸ���ͼ����֪ƽ��ʱSO2��ת����Ϊ0.80���������ת����SO2�����ʵ����������α�ʾ��ƽ��ʱ�����ʵ����ʵ�����������ƽ��Ũ�ȴ���ƽ�����ʽ���㣮
��ת����SO2Ϊ��2mol��0.80=1.6mol��
������ƽ��Ϊ��Чƽ�⣬�¶Ȳ���ƽ�ⳣ�����䣬��������⣮
��2�����ø�˹������Ŀ�귽��ʽ���Ȼ�ѧ����ʽ��CH4��g��+4NO2��g��=4NO��g��+C02��g��+2H20��g����H=-574kJ•mol-1 ��
CH4��g��+4NO��g��=2N2��g��+CO2��g��+2H20��g����H=-1160kJ•mol-1��
���ݸ�˹���ɣ�������ʽ���Σ���+�ڣ���$\frac{1}{2}$�ã�CH4��g��+2NO2��g��=N2��g��+CO2��g��+2H20��g����H=-867KJ•mol-1
����Ȼ�ѧ����ʽ������ϵ�����״����4.48L CH4��ԭNO2��N2���������У��ų���������
��� �⣺��1���ٸ���ͼ����ת����SO2Ϊ��2mol��0.80=1.6mol
2SO2��g��+O2��g��$\frac{\underline{����}}{��}$2SO3��g��
n0 ��mol�� 2.0 1.0 0
��n��mol�� 1.6 0.8 1.6
nƽ��mol�� 0.4 0.2 1.6
�������Ϊ10L�������ƽ��Ũ�ȣ�C��SO2��=0.04mol/L C��O2��=0.02mol/L C��SO3��=0.16mol/L
K=$\frac{{c}^{2}��S{O}_{3}��}{{c}^{2}��S{O}_{2}��c��{O}_{2}��}$=$\frac{0.1{6}^{2}}{0.0{4}^{2}��0.02}$=800��
�ʴ�Ϊ��800��
������ƽ��Ϊ��Чƽ�⣬�ﵽƽ�������ʵ����ʵ�����ͬ��
2SO2��g��+O2��g�� $\frac{\underline{����}}{��}$2SO3��g��
n0 ��mol�� 1.0 0.5 a
��n��mol�� 0.6 0.3 0.6
nƽ��mol�� 0.4 0.2 1.6
a=1.0��
SO2ƽ��ת����=$\frac{������}{��ʼ��}$��100%=$\frac{0.6}{1.0}$��100%=60%��
�ʴ�Ϊ��1.0��60%��
���¶Ȳ����K���䣬�ʴ�Ϊ��=��
��2��CH4��g��+4NO2��g��=4NO��g��+C02��g��+2H20��g����H=-574kJ•mol-1 ��
CH4��g��+4NO��g��=2N2��g��+CO2��g��+2H20��g����H=-1160kJ•mol-1��
���ݸ�˹���ɣ�������ʽ���Σ���+�ڣ���$\frac{1}{2}$�ã�CH4��g��+2NO2��g��=N2��g��+CO2��g��+2H20��g����H=-867KJ•mol-1
n��CH4��=$\frac{4.48L}{22.4L/mol}$=0.2mol
CH4��g��+2NO2��g��=N2��g��+CO2��g��+2H20��g����H=-867KJ•mol-1��8e-
1 867KJ 8
0.2mol 173.4KJ 1.6mol
���Էų�������Ϊ173.4KJ��
�ʴ�Ϊ��173.4��
���� ���⿼���˻�ѧƽ��ͼ��ѧƽ�ⳣ������ѧƽ��ļ��㡢��˹���ɣ���Ŀ���֪ʶ��࣬�Ѷ��еȣ�����ʱע�⿴��ͼ������ʽ��ƽ������Ӧ�ã�

 һ����������ϵ�д�
һ����������ϵ�д�| A�� | �綾Ʒ | B�� | ��ȼҺ�� | C�� | ������ | D�� | ��ʴƷ |
| A�� | �������백ˮ��Ӧ��Al3++3OH-�TAl��OH��3�� | |
| B�� | ̼������ڴ��CaCO3+2H+�TCa2++H2O+CO2�� | |
| C�� | ����NaHSO4��Һ������Ba��OH��2��Һ��Ӧ��2H++SO42-+Ba2++2OH-�TBaSO4��+2H2O | |
| D�� | NaHCO3��Һ�м�������������Һ��OH-+HCO3-�TCO2��+H2O |
| ������ | K+ Na+ Cu2+ Al3+ |
| ������ | SO42- HCO3-OH- Cl- |
�ٽ���������ˮ��DΪ��ɫ��Һ��������Ϊ��ɫ��Һ��
�ڽ�E��Һ���뵽C��Һ�г��ְ�ɫ�����������μӣ������ܽ⣻
�۽�����ɫ��Ӧ������BΪ��ɫ������ɫ�ܲ�������
���ڸ���Һ�м��������ữ�����ᱵ��Һ��ֻ��A�зų���ɫ���壬ֻ��D�в�����ɫ������
�ݽ�B��C����Һ��ϣ�δ���������������ɣ���������ʵ����գ�
��1��д��B��D�Ļ�ѧʽ��BKCl��DCuSO4��
��2��C��������ˮ���������ӷ���ʽ��ʾ�侻ˮԭ��Al3++3H2O�TAl��OH��3+3H+��
��3������0.01mol A����Һ�뺬0.02mol E����Һ��Ӧ������Һ�еμ�0.1mol•L-1ϡ���ᣮ��ͼͼ������ȷ��ʾ������������������CO2�����ʵ����Ĺ�ϵ����C��

��4����mmL bmol•L-1 C��Һ�У���������a mol•L-1��E��Һ����a��3bʱ�����ɳ��������ʵ�����$\frac{am}{3000}$mol����3b��a��4b��ʱ�����ɳ��������ʵ����ǣ�4bm-am����10-3mol��

| A�� | ͼ�ױ�ʾlmol H2��g����ȫȼ������ˮ��������241.8kJ���� | |
| B�� | ͼ�ױ�ʾ2mol H2��g�������е�������2mol H2O��g�������е�������483.6kJ | |
| C�� | ͼ�ұ�ʾ������ϡ��HA��HB�������ͼ�ó����ۣ�HA�����Դ���HB������ | |
| D�� | ͼ������ʼʱHA�����ʵ���Ũ�ȴ���HB |
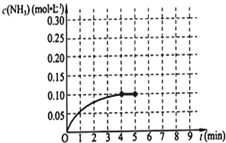 ��һ�̶��ݻ�Ϊ2L���ܱ������ڼ���0.2mol��N2��0.6mol��H2����һ�������·������·�Ӧ��N2��g��+3H2��g��?2NH3��g������H��0����Ӧ��NH3�����ʵ���Ũ�ȵı仯�����ͼ��ʾ����ش��������⣺
��һ�̶��ݻ�Ϊ2L���ܱ������ڼ���0.2mol��N2��0.6mol��H2����һ�������·������·�Ӧ��N2��g��+3H2��g��?2NH3��g������H��0����Ӧ��NH3�����ʵ���Ũ�ȵı仯�����ͼ��ʾ����ش��������⣺