��Ŀ����
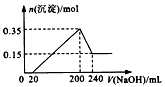
 ��һ��������þ�����Ļ����Ͷ��100mL�����У�����ȫ���ܽ����������Һ�м���NaOH��Һ�����ɳ��������ʵ��������NaOH��Һ�������ϵ��ͼ��ʾ���������ǽ��������ᷴӦʱHCl�Ļӷ���������˵����ȷ���ǣ�������
��һ��������þ�����Ļ����Ͷ��100mL�����У�����ȫ���ܽ����������Һ�м���NaOH��Һ�����ɳ��������ʵ��������NaOH��Һ�������ϵ��ͼ��ʾ���������ǽ��������ᷴӦʱHCl�Ļӷ���������˵����ȷ���ǣ���������������ͼ���п������ӿ�ʼ������NaOH��Һ20mL��û�г������ɣ�˵��ԭ��Һ�������ܽ�Mg��Al����ʣ�࣬��ʱ�����ķ�ӦΪ��HCl+NaOH=NaCl+H2O����V��NaOH��Һ��=200mLʱ�����������ʱΪMg��OH��2��Al��OH��3��
��V��NaOH��Һ��=240mLʱ���������ټ��٣���ʱȫ��ΪMg��OH��2��
��200mL��240mL��NaOH�ܽ�Al��OH��3��NaOH+Al��OH��3=NaAlO2+2H2O��
���ݸ��θ����ʵ������м�����жϣ�
��V��NaOH��Һ��=240mLʱ���������ټ��٣���ʱȫ��ΪMg��OH��2��
��200mL��240mL��NaOH�ܽ�Al��OH��3��NaOH+Al��OH��3=NaAlO2+2H2O��
���ݸ��θ����ʵ������м�����жϣ�
����⣺��ͼ���п������ӿ�ʼ������NaOH��Һ20mL��û�г������ɣ�˵��ԭ��Һ�������ܽ�Mg��Al����ʣ�࣬��ʱ�����ķ�ӦΪ��HCl+NaOH=NaCl+H2O����V��NaOH��Һ��=200mLʱ�����������ʱΪMg��OH��2��Al��OH��3��
A����V��NaOH��Һ��=240mLʱ���������ټ��٣���ʱȫ��ΪMg��OH��2����n��Mg��=n[Mg��OH��2]=0.15mol��
m��Mg��=0.15mol��24g?mol-1=3.6g��
n��Al��=n[Al��OH��3]=0.35mol-0.15mol=0.2mol��
m��Al��=0.2mol��27g?mol-1=5.4g������þ������������Ϊ9g����A��ȷ��
B������þ���������ʵ�����֪��Ӧ����MgCl2��AlCl3��Ҫ���0.15mol��2+0.2mol��3=0.9mol������ʱ����������������Ũ��c��HCl����
=9mol/L����B����
C����200mL��240mL��NaOH�ܽ�Al��OH��3��NaOH+Al��OH��3=NaAlO2+2H2O����˹�������n��NaOH��=n[Al��OH��3]=0.2mol����c��NaOH��=0.2 mol0.04 L=5mol?L-1����C��ȷ��
D������Mg+2HCl=MgCl2+H2����2Al+6HCl=2AlCl3+3H2�����Լ��������n��H2��=0.45mol����״����V��H2��=0.45mol��22.4L?mol-1=10.08L����D����
��ѡAC��
A����V��NaOH��Һ��=240mLʱ���������ټ��٣���ʱȫ��ΪMg��OH��2����n��Mg��=n[Mg��OH��2]=0.15mol��
m��Mg��=0.15mol��24g?mol-1=3.6g��
n��Al��=n[Al��OH��3]=0.35mol-0.15mol=0.2mol��
m��Al��=0.2mol��27g?mol-1=5.4g������þ������������Ϊ9g����A��ȷ��
B������þ���������ʵ�����֪��Ӧ����MgCl2��AlCl3��Ҫ���0.15mol��2+0.2mol��3=0.9mol������ʱ����������������Ũ��c��HCl����
| 0.9mol |
| 0.1L |
C����200mL��240mL��NaOH�ܽ�Al��OH��3��NaOH+Al��OH��3=NaAlO2+2H2O����˹�������n��NaOH��=n[Al��OH��3]=0.2mol����c��NaOH��=0.2 mol0.04 L=5mol?L-1����C��ȷ��
D������Mg+2HCl=MgCl2+H2����2Al+6HCl=2AlCl3+3H2�����Լ��������n��H2��=0.45mol����״����V��H2��=0.45mol��22.4L?mol-1=10.08L����D����
��ѡAC��
���������⿼��þ������Ҫ�������ͼ�������ʽ���飬��Ŀ�Ѷ��еȣ�ע�����ͼ����ε����ʵ����Ĺ�ϵ�����ݸ��εĻ�ѧ��Ӧ�����÷���ʽ���㣮

��ϰ��ϵ�д�
�����Ŀ
��һ��������þ�����Ļ����Ͷ��100ml�����У�����ȫ���ܽ����������Һ�м���NaOH��Һ�����ɳ��������ʵ��������NaOH��Һ�������ϵ��ͼ��ʾ���������ǽ��������ᷴӦʱHCl�Ļӷ���������˵����ȷ����
| A��þ������������Ϊ9g |
| B����������ʵ���Ũ��Ϊ5 mol��L��1 |
| C��NaOH��Һ�����ʵ���Ũ��Ϊ5 mol��L��1 |
| D�����ɵ������ڱ�״���µ����Ϊ11.2 L |
 ��һ��������þ�����Ļ����Ͷ��100mL�����У�����ȫ���ܽ����������Һ�м���NaOH��Һ�����ɳ��������ʵ��������NaOH��Һ�������ϵ��ͼ��ʾ���������ǽ��������ᷴӦʱHCl�Ļӷ���������˵����ȷ���ǣ�������
��һ��������þ�����Ļ����Ͷ��100mL�����У�����ȫ���ܽ����������Һ�м���NaOH��Һ�����ɳ��������ʵ��������NaOH��Һ�������ϵ��ͼ��ʾ���������ǽ��������ᷴӦʱHCl�Ļӷ���������˵����ȷ���ǣ�������