��Ŀ����
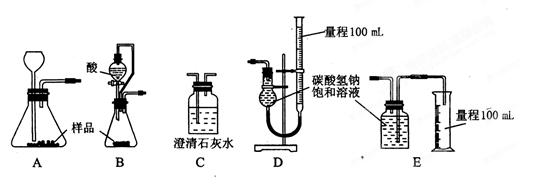
��һ������̼�������ʵ�̼��������Ʒ��Ϊ�˲ⶨ�䴿�ȣ�ijУ����ѧϰС����ʵ����������ʵ�飺��1����һ��ͬѧ��ȡ��Ʒm
��Ϊ��ɲⶨ������ͼʾ�л�ȱ�ٵ�����������_______________________��
��Ϊ�˼���ʵ�����ڼ��������صĹ�����Ӧ��β�����
___________________________________________________________________��
������������Ϊm
��2���ڶ���ͬѧ��ȡ��Ʒm g�����������ͼ��ʾ��װ�ã�
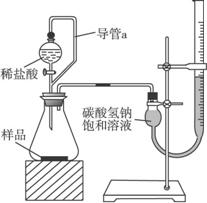
��װ���е���a��������________________________________________________________��
��ʵ�����ʱ������ͬѧ�ڲ���ʵ���������������ʱӦע����Щ���⣿
____________________________________________________________________
____________________________________________________________________��
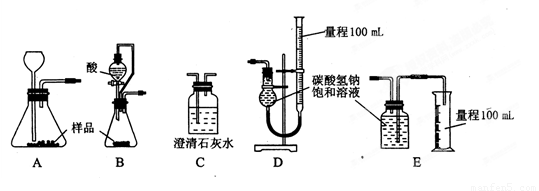
��3��������������ʵ�鷽��������Ϊ����ʵ�鷽��������СЩ��
____________________________________________________________________��
��4���������һ�ֳ�����ʵ��ԭ������ķ�����д����Ӧ�Ļ�ѧ����ʽ��
____________________________________________________________________��
��1��������ǯ��������
�ڼ��ȺŸ���������ȴ�����º�������ظ�������ֱ���������γ�����������С��0.1 g
��![]()
��2����ʹ��Һ©��������ѹǿ����ƿ������ѹǿ��ȣ�ʹ��Һ©���е�ϡ������˳������
�ڴ�ʵ��װ����ȴ�������ƶ��ζ��ܣ�ʹ��Һ������������Һ����ƽ���ٽ�������ζ�����ˮ�İ�Һ����ʹ���ƽ����ȡҺ����������õ������ڸ�״��ʱ�����
��3���ڶ���Ϻ�
��4��BaCl2+Na2CO3====BaCO3��+2NaCl�����������𰸾��ɣ�
����������Ŀ�Ǹ��ۺ�ʵ�鿼���⣬��һ���ѶȺ����ֶȡ������˼��Ȳ�����ʶ���ټ����õ�������������ȴʱҪ�ڸ���������ȴ��������ʱ�����IJ�����ʶ���ζ��ܵ�Һ���������ܵ�Һ��Ҫƽ��������Һ��ҲҪƽ���ܻ������˳��������صķ�����ʵ�����ݵĴ����Լ���ʵ�鷽���������ۺ���ơ������Щ����Ĺؼ������ջ�ѧʵ��Ļ���������ʶ��ͬʱ��Ҫ����Na2CO3��NaHCO3�����ʵ����ʡ�

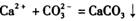 �ķ�Ӧ��������·���:��ȡ��Ʒ�������Һ����������ŨCaCl2��Һ����ַ�Ӧ����ˡ�ϴ�ӡ���������������㴿�ȡ�
�ķ�Ӧ��������·���:��ȡ��Ʒ�������Һ����������ŨCaCl2��Һ����ַ�Ӧ����ˡ�ϴ�ӡ���������������㴿�ȡ�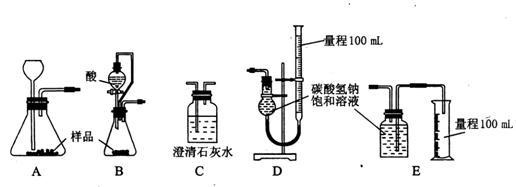
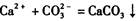 �ķ�Ӧ��������·���:��ȡ��Ʒ�������Һ����������ŨCaCl2��Һ����ַ�Ӧ����ˡ�ϴ�ӡ���������������㴿�ȡ�
�ķ�Ӧ��������·���:��ȡ��Ʒ�������Һ����������ŨCaCl2��Һ����ַ�Ӧ����ˡ�ϴ�ӡ���������������㴿�ȡ�