��Ŀ����
������ͼ��ʵ��װ�ÿ��Խ����Ƹ�ˮ�ķ�Ӧʵ�飬�����ռ����������ɵ����塣Na���ܶ�Ϊ0.97 g��cm-3��ú�͵��ܶ�Ϊ0.87 g��cm-3�� 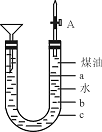
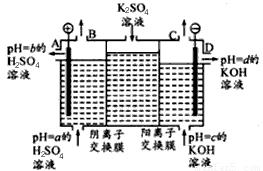
��ش��������⡣
��1��Һ������������Ϻرջ��������ұ߽�������ú���м���һС���ƣ��������ý�������Ӧ��ʼǰ�Ƶ�λ����_____________�����a����b����c������
��2����Ӧ������ú�Ͳ��ˮ�����֮��������������Ӧƽ���������С���˵���������������?ԭ��_______________________________________��
��3��д��Na��ˮ��Ӧ�����ӷ���ʽ____________��
��4��װ���е�©���ڷ�Ӧ�����е���Ҫ������__________________________________��
��5����ʵ�����ʹ��Ӧƽ���������С��ռ������������⣬����һ���ŵ���____________��������Ϊ_____________________________________________________________________��
��������������2�ı仯��Ǩ�ƣ�֪ʶ����Ȼ������ˮ�ķ�Ӧ��
�𰸣���1��a����2�����Ƹ�ˮ��Ӧ����ʱ��H2������Na����ú�Ͳ��У���Ӧֹͣ������H2�������٣�Na����ú�Ͳ��ˮ��Ľ��棬��Ӧ�ֽ���
��3��2NaOH+2H2O====2Na++2OH-+H2��
��4����ֹˮ���
��5����ȫ���˷�Ӧ���ڸ��������½��е�

|
����ʵ������淶���ܴﵽʵ��Ŀ�ĵ��� | |
| [����] | |
A�� |
��ȡһ��������NaCl����ֽ��С�ĵؼ��뵽����ƿ�У�Ȼ������Ͳ��ȡһ������ˮ��������ƿ�У�����ȷŨ�ȵ�NaCl��Һ |
B�� |
ʵ����������ͼ��ʾװ�ÿ���ȡ��ϩ����֤��ϩ��ijЩ��ѧ����
|
C�� |
��������ϡ������MnO2�ڼ��������£����Ƶ����� |
D�� |
�ó����ʯ��ˮ��Һ������̼���ơ�̼��������Һ |