��Ŀ����
ѡ����
����������������ѡһ������
1.��������ǻ�ѧ�о����ȵ�֮һ����ѧ�����ú�����ऻ�����वĽṹʽΪ![]() �������������ڱ����Ļ��������Ϊ�м��壬ʵ����ѭ�������⣬ʾ��ͼ���£����г����ַ�Ӧ��������
�������������ڱ����Ļ��������Ϊ�м��壬ʵ����ѭ�������⣬ʾ��ͼ���£����г����ַ�Ӧ��������

��1���������ķ���ʽΪ________________________________________��
��2���������ϳɷ������£���Ӧ�����ԣ����������Ľṹδ�����⣬��Ӧʽ����ƽ����
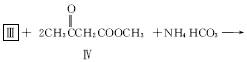
![]()
��������������_______________________��
��3���û���������ṹʽ����ͼ���������ԭ�ϣ�Ҳ�ܽ������Ƶ�������Ӧ�������л�����ĽṹʽΪ____________________��

��4������˵����ȷ����________________������ĸ����
A.��������������Ǽ��ǻ�����ȩ����2�ǻ�����ȩ
B.���������л�ԭ�ԣ�����������ԣ����ܱ����Ը��������Һ����
C.����������ɷ���ˮ�ⷴӦ
D.������������Ȼ�����ɫ�����ɷ���������Ӧ��������������ԭ��Ӧ
��5����़���������Ϊ�������ӵ���ȡ����2��़����������������Ľṹʽ����ͼ����ϳ�ԭ��2��़���ĽṹʽΪ__________________������ͬ���칹���У���ऻ���ֻ��һ����ԭ�ӱ�ȡ������़�������ͬ���칹����____________________�֡�

��
2.ͭ���ʼ��仯�����ںܶ���������Ҫ��;�������ͭ����������ߵ��£���ˮ����ͭ������ɱ������
��1��Cuλ��Ԫ�����ڱ��ڢ�B�塣Cu2+�ĺ�������Ų�ʽΪ_____________________��
��2����ͼ��ͭ��ij��������ľ����ṹʾ��ͼ����ȷ���þ����������ӵĸ���Ϊ________��

��3������CuSO4��5H2O��д�ɣ�Cu��H2O��4��SO4��H2O����ṹʾ��ͼ���£�

����˵����ȷ����_________________������ĸ����
A.�������ṹʾ��ͼ�У�������ԭ�Ӷ�����sp3�ӻ�
B.�������ṹʾ��ͼ�У�������λ�������ۼ������Ӽ�
C.�����Ƿ��Ӿ��壬���Ӽ�������
D.�����е�ˮ�ڲ�ͬ�¶��»�ֲ�ʧȥ
��4��������ͭ��Һ�м��������ˮ�������ɣ�Cu��NH3��4��2+�����ӡ���֪NF3��NH3�Ŀռ乹�Ͷ��������Σ���NF3������Cu2+�γ������ӣ���ԭ����_____________________��
��5��Cu2O���۵��Cu2S��______________����ߡ��͡����������ԭ��____________________________��
@@1. ��1��C11H13NO4 ��2����ȩ
��3��
��4��BC
��5��![]() 12
12
1. ��1��C11H13NO4 ��2����ȩ
��3��
��4��BC
��5��![]() 12
12
��������1��ȷ������ʽʱ��ע��̼��4�ۡ�
��2����ķ���ʽΪC11H15NO5����ԭ���غ���֪��ķ���ʽΪCH2O2��Ϊ���ᡣ
��3���ԱȢ��Ľṹ���ɵõ���Ӧʵ�ʣ��Ӷ�д������Ľṹʽ��
��5���۲���Ľṹ��ȥ�������֣�����2��़���Ľṹ��ʽ��������4�֣����Է�������Ģ���ͬ���칹����4��3��12�֡�
2.��1��1s22s22p63s23p63d9���Ar��3d9
��2��4
��3��BD
��4��F�ĵ縺�Ա�N��N��F�ɼ����Ӷ���Fƫ�ƣ�����NF3��Nԭ�Ӻ˶���¶Ե��ӵ�����������ǿ�������γ���λ������NF3������Cu2+�γ�������
��5����
Cu2O��Cu2S��ȣ���������ͬ���������������Ҳ��ͬ����O2-�İ뾶��S2-С������Cu2O�ľ����ܸ����۵����
��������1��Cuԭ�ӳ�ΪCu2+��ʧȥ�������ӡ�
��2��ȷ������Ŀ��ע��������
��3���ɽṹʾ��ͼ֪�����������в��������Ӽ�������ʱ�ֲ�ʧH2O��
��5�����Ӿ����ۡ��е�ߵͱȽ��У�Ҫͨ�����Ӱ뾶�Ĵ�С�Ƚϼ���ǿ�����Ӷ�ȷ���ۡ��е�ߵ͡�
