��Ŀ����
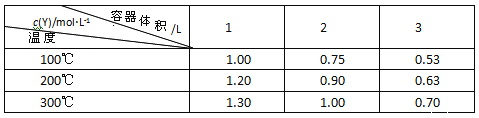
����Ŀ��T ��ʱ���мס��������ܱ������������������Ϊ1 L�������������Ϊ2 L���ֱ�������������м���6 mol A��3 mol B��������Ӧ���£�3A(g)��bB(g)![]() 3C(g)��2D(g)����H��0,4 minʱ�������ڵķ�Ӧǡ�ôﵽƽ�⣬A��Ũ��Ϊ2.4 mol��L��1��B��Ũ��Ϊ1.8 mol��L��1��t minʱ�������ڵķ�Ӧ�ﵽƽ�⣬B��Ũ��Ϊ0.8 mol��L��1�����������Ϣ�ش��������⣺
3C(g)��2D(g)����H��0,4 minʱ�������ڵķ�Ӧǡ�ôﵽƽ�⣬A��Ũ��Ϊ2.4 mol��L��1��B��Ũ��Ϊ1.8 mol��L��1��t minʱ�������ڵķ�Ӧ�ﵽƽ�⣬B��Ũ��Ϊ0.8 mol��L��1�����������Ϣ�ش��������⣺
��1���������з�Ӧ��ƽ������v(B)��_______________����ѧ����ʽ�м�����b��_________��
��2���������з�Ӧ�ﵽƽ������ʱ��t_______4 min(������������С��������������)��ԭ����___________________________________________________��
��3��T ��ʱ������һ�����������ͬ�ı������У�Ϊ�˴ﵽƽ��ʱB��Ũ����ȻΪ0.8 mol��L��1����ʼʱ����������м���C��D�����ʵ����ֱ�Ϊ3 mol��2 mol���������A��B�����ʵ����ֱ���__________��__________��
��4����Ҫʹ�ס���������B��ƽ��Ũ����ȣ����Բ�ȡ�Ĵ�ʩ��________��
A�������¶Ȳ��䣬����������������2 L
B����������������䣬ʹ�����������¶�
C����������ѹǿ���¶ȶ����䣬����м���һ������A����
D����������ѹǿ���¶ȶ����䣬����м���һ������B����
���𰸡�0.3 mol��L��1��min��11������������������ڼ������������Ũ����Խ�С����Ӧ���ʽ�������ƽ������ʱ�䳤3 mol2 molA��C
��������
(1)�����������Ϊ1L������6molA��3molB����A��B��Ũ�ȷֱ�Ϊ��6mol/L��3mol/L��4minʱ�������ڵķ�Ӧǡ�ôﵽƽ�⣬A��Ũ��Ϊ2.4mol/L��B��Ũ��Ϊ1.8mol/L����A��B��Ũ�ȱ仯Ϊ����c(A)=6mol/L-2.4mol/L=3.6mol/L��B��Ũ�ȱ仯Ϊ����c(B)=3mol/L-1.8mol/L=1.2mol/L����ʱ����ƽ������v(B)=![]() =0.3 molL-1min-1��Ũ�ȱ仯�뻯ѧ�����������ȣ���3��b=��c(A)����c(B)=3.6mol/L��1.2mol/L=3��1����b=1���ʴ�Ϊ��0.3 molL-1min-1��1��
=0.3 molL-1min-1��Ũ�ȱ仯�뻯ѧ�����������ȣ���3��b=��c(A)����c(B)=3.6mol/L��1.2mol/L=3��1����b=1���ʴ�Ϊ��0.3 molL-1min-1��1��
(2)�����������Ϊ1L�������������Ϊ2L����������������ڼģ�Ũ��С��Ӧ����С������ƽ���ʱ�䳤�������������з�Ӧ�ﵽƽ��ʱ����ʱ�����4min���ʴ�Ϊ�����ڣ� ��������������ڼ��������������Ӧ��Ũ�ȼ�С����Ӧ���ʼ������ﵽƽ������Ҫ��ʱ���Ҫ����
(3)���ݵ�Чƽ����ɣ��ں��º����£�ת��Ϊͬһ��Ӧ��������ʵ����ʵ�����ԭ����Ӧ��ȼ��ɣ�3molC��2molD��ȫת��������3molA��1molB������3molA��2molB��������Ϊ��Чƽ�⣬�ʴ�Ϊ��3 mol��2 mol��
(4)���ڸ÷�Ӧ���������Ŀ��淴Ӧ�����ڼ��������С����ѹǿ��ƽ�����������ƶ������з�Ӧ���ת����С���ң��Ҵﵽƽ��ʱ���и����Ũ�ȶ������ҵġ�A�������¶Ȳ��䣬����������������2L�����ʱ����Ϊ��Чƽ�⣬�ס���������B��ƽ��Ũ����ȣ���A��ȷ��B����������������䣬ʹ�����������¶ȣ��÷�ӦΪ���ȷ�Ӧ��ƽ�����������ƶ�������BŨ��������������ס���������B��ƽ��Ũ����ȣ���B����C����������ѹǿ���¶ȶ����䣬����м���һ������A���壬���ڷ�Ӧ��A��Ũ��������ƽ�����������ƶ�������B��Ũ�ȼ�С������ʵ�ּס���������B��ƽ��Ũ����ȣ���C��ȷ��D����������ѹǿ���¶ȶ����䣬����м���һ������B���壬�ﵽƽ��ʱ����B��Ũ�ȸ���������ס���������B��ƽ��Ũ����ȣ���D���ʴ�Ϊ��AC��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�