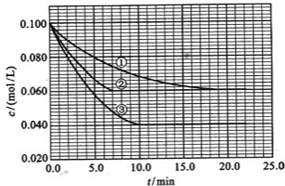
��Ŀ����
��7�֣�������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ���ش��������⣺

(1)T�����ڱ��е�λ�� __________������������Rͬ����Ԫ�ص�ԭ������Ϊ ����Ԫ��Ϊ Ԫ�أ���������ǽ�������
(2)Ԫ�صķǽ�����(ԭ�ӵõ��ӵ�����)��Q________T(�ǿ�ڡ������ڡ�)��
(3)W�ĵ�����������������ˮ����Ũ��Һ�����ܷ�����Ӧ�������������ʣ�����һ�������壬��Ӧ�Ļ�ѧ����ʽΪ______________________________________________��
(4)R�ж�����������м���Է���������С����һ�������£�2 L�ļ�������0.5 L���������ϣ����û�����屻������NaOH��Һ��ȫ���պ�û����������������ɵ� R�ĺ�������ֻ��һ�֣���ú������εĻ�ѧʽ��____________��
������������Ԫ�������ڱ��е�λ�ÿ�֪T���ڵ������ڣ�T��������������������������ȣ�����T��Al����Q��C��R��N��W��S��
��1��N�ǵڢ�A�����Ե������ڡ��ڢ�AԪ�ص�ԭ��������7��8��18��18��32��83����Ԫ����Ǧ�����ڽ���Ԫ�ء�
��2��C�Ƿǽ�����Al�ǽ�����ǰ�ߵķǽ�����ǿ�ں��ߡ�
��3��������Ļ��ϼ���0�ۣ�Ũ��������Ļ��ϼ��ǣ�6�ۣ������߷�Ӧ��������ֻ��2�֣���һ�������壬��Ϊ������һ����ˮ���������ֻ����SO2��
��4��N������������Է����������µ���NO���ڷ�Ӧ��NO��O2�����ʵ���֮����4�U1�����ݵ�ʧ�����غ��֪NO��ʧȥ1�����ӣ����ϼ��ɣ�2�����ߵ���3�ۣ���˺���������NaNO2��
(1) �������� IIIA�� 83������ (2)ǿ��
(3)
S+2H2SO4(Ũ) 3SO2����2H2O (4) NaNO2
3SO2����2H2O (4) NaNO2

 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�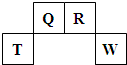 ��2009?������������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ�
��2009?������������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ�
 ������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ�
������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ�
 ������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ������ͼ��ʾ������Ԫ��T��������������������������ȣ���ش��������⣺
������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ������ͼ��ʾ������Ԫ��T��������������������������ȣ���ش��������⣺
 ��2013?��خ��ģ�⣩������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ������ƶ���ȷ���ǣ�������
��2013?��خ��ģ�⣩������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ������ƶ���ȷ���ǣ�������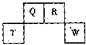 ��2012?������һģ��������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ���ش��������⣺
��2012?������һģ��������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ���ش��������⣺