��Ŀ����
ʹ��22.4 L/molʱӦע�����������������ж�����˵���Ƿ���ȷ���粻��ȷ�������
(1)1 mol CO2�����ԼΪ22.4 L_______________��
(2)�ڱ�״���£�1 mol CO2��1 mol CO�����ķ�������ͬ����ռ�����Ҳ��ͬ___________��
(3)�ڱ�״���£�O2�����ԼΪ22.4 L_______________��
(4)1 mol H2��20��ʱ���һ������22.4 L______________��
(5)1 mol CO2��CO�Ļ�������ڱ�״���£���ռ���ԼΪ22.4 L_____________��
(1)1 mol CO2�����ԼΪ22.4 L_______________��
(2)�ڱ�״���£�1 mol CO2��1 mol CO�����ķ�������ͬ����ռ�����Ҳ��ͬ___________��
(3)�ڱ�״���£�O2�����ԼΪ22.4 L_______________��
(4)1 mol H2��20��ʱ���һ������22.4 L______________��
(5)1 mol CO2��CO�Ļ�������ڱ�״���£���ռ���ԼΪ22.4 L_____________��
(1)����ȷ���ڱ�״���£�1 mol CO2�����ԼΪ 22.4 L��
(2)��ȷ��
(3)����ȷ���ڱ�״���£�1mol O2�����ԼΪ22.4 L��
(4)����ȷ��1 mol H2��20��ѹǿ��ȷ��ʱ����������ܴ���22.4 L������22.4 L��С��22.4 L��
(5)��ȷ��
(2)��ȷ��
(3)����ȷ���ڱ�״���£�1mol O2�����ԼΪ22.4 L��
(4)����ȷ��1 mol H2��20��ѹǿ��ȷ��ʱ����������ܴ���22.4 L������22.4 L��С��22.4 L��
(5)��ȷ��

��ϰ��ϵ�д�
�����Ŀ
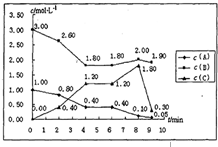 ��һ���ݻ��̶�Ϊ1L���ܱ������У�������ӦmA��g��+nB��g���TpC��g����H=������Ӧ�������ͼ��ʾ��
��һ���ݻ��̶�Ϊ1L���ܱ������У�������ӦmA��g��+nB��g���TpC��g����H=������Ӧ�������ͼ��ʾ��