��Ŀ����
[s1] ��ͼ��ʵ������SO2����֤SO2ijЩ���ʵ�װ��ͼ��
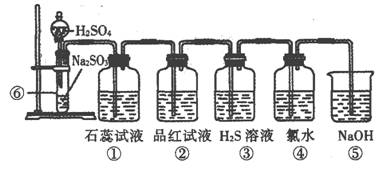
�Իش�
(1)���з����Ļ�ѧ��Ӧ����ʽΪ��____________________________________��
(2)����ʵ������Ϊ________________��֤��SO2��ˮ��Ӧ����Һ��_____________��
(3)����Ʒ����Һ����Ϊ______________________��֤��SO2��____________�ԡ�
(4)����������_____________________��֤��SO2��_________�ԡ�
��Ӧ�Ļ�ѧ����ʽΪ ��
(5)����������_____________________��֤��SO2��_________�ԡ�
��Ӧ�����ӷ���ʽΪ ��
(6)�ݵ�������_____________________����Ӧ�����ӷ���ʽΪ��____________________��
[s1]28��
[s1] ����ͼװ�òⶨˮ���⡢��Ԫ�ص������ȣ��䷽���Ƿֱ�ⶨͨ����ǰ�����ܵ��������U�ܵ������ʵ����m��H����m��O����1��8�����жԵ�����һ�����ԭ��ķ����У�һ���������� �� ��
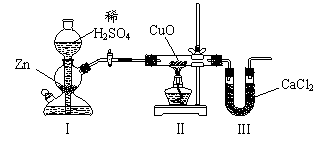 |
A����װ��֮��ȱ�ٸ���װ�� B��IIIװ�ú�ȱ�ٸ���װ��
C����װ���в���������ˮ���� D��CuOû��ȫ������ԭ
[s1]16��

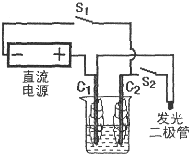 ȼ�ϵ����һ�ֽ���ѧ��Ӧ����������ֱ��ת���ɵ��ܵ�װ�ã�ij�о���ѧϰС����һ�λ�У�������ͼ��װʵ��װ�ã��������������̼��Ϊ�缫���������Һ��0.5mol?L-1Na2SO4��Һ����Դ��3��6Vֱ����Դ�������������Ե�ѹΪ1.7V������Ϊ0.6mA��
ȼ�ϵ����һ�ֽ���ѧ��Ӧ����������ֱ��ת���ɵ��ܵ�װ�ã�ij�о���ѧϰС����һ�λ�У�������ͼ��װʵ��װ�ã��������������̼��Ϊ�缫���������Һ��0.5mol?L-1Na2SO4��Һ����Դ��3��6Vֱ����Դ�������������Ե�ѹΪ1.7V������Ϊ0.6mA��
