��Ŀ����
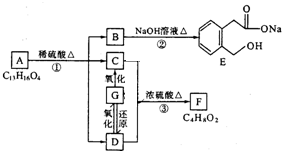 ͼ��A��B��C��D��E��F��G��Ϊ�л������B������ֻ��һ����״�ṹ������ͼʾ�ش����⣺
ͼ��A��B��C��D��E��F��G��Ϊ�л������B������ֻ��һ����״�ṹ������ͼʾ�ش����⣺��1��B�ķ���ʽ��
C9H10O3
C9H10O3
��A�Ľṹ��ʽ��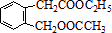
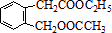
ˮ��
ˮ��
����2����Ӧ�۵Ļ�ѧ����ʽ��
CH3COOH+C2H5OH
CH3COOC2H5+H2O
| Ũ���� |
| �� |
CH3COOH+C2H5OH
CH3COOC2H5+H2O
��| Ũ���� |
| �� |
��3������G���ʵ��йػ�ѧ����ʽΪ
CH3CHO+2Ag��NH3��2OH
CH3COONH4+2Ag��+3NH3+H2O
| �� |
CH3CHO+2Ag��NH3��2OH
CH3COONH4+2Ag��+3NH3+H2O
��д1�����ɣ�| �� |
��4����������3��������B��ͬ���칹�����Ŀ��
3
3
���ٺ����ڶ�ȡ�������ṹ
����B����ͬ������
�۲���FeCl3��Һ������ɫ��Ӧ
д����������һ��ͬ���칹��Ľṹ��ʽ

��������E�Ľṹ������֪��BΪ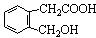 �����F�ķ���ʽC4H8O2��C��Dת��ΪF�ķ�Ӧ����������֪CΪ���ᣬDΪ�Ҵ���GΪ��ȩ��FΪ������������BCD�Ľṹ�����A�ķ���ʽ��Aת��ΪBCD�ķ�Ӧ���������Ƴ�AΪ
�����F�ķ���ʽC4H8O2��C��Dת��ΪF�ķ�Ӧ����������֪CΪ���ᣬDΪ�Ҵ���GΪ��ȩ��FΪ������������BCD�Ľṹ�����A�ķ���ʽ��Aת��ΪBCD�ķ�Ӧ���������Ƴ�AΪ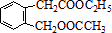 ����ϣ��л���Ľṹ�������Լ���ĿҪ��ͽ�����
����ϣ��л���Ľṹ�������Լ���ĿҪ��ͽ�����
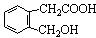 �����F�ķ���ʽC4H8O2��C��Dת��ΪF�ķ�Ӧ����������֪CΪ���ᣬDΪ�Ҵ���GΪ��ȩ��FΪ������������BCD�Ľṹ�����A�ķ���ʽ��Aת��ΪBCD�ķ�Ӧ���������Ƴ�AΪ
�����F�ķ���ʽC4H8O2��C��Dת��ΪF�ķ�Ӧ����������֪CΪ���ᣬDΪ�Ҵ���GΪ��ȩ��FΪ������������BCD�Ľṹ�����A�ķ���ʽ��Aת��ΪBCD�ķ�Ӧ���������Ƴ�AΪ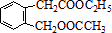 ����ϣ��л���Ľṹ�������Լ���ĿҪ��ͽ�����
����ϣ��л���Ľṹ�������Լ���ĿҪ��ͽ���������⣺��E�Ľṹ������֪��BΪ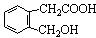 �����F�ķ���ʽC4H8O2��C��Dת��ΪF�ķ�Ӧ����������֪CΪ���ᣬDΪ�Ҵ���GΪ��ȩ��FΪ������������BCD�Ľṹ�����A�ķ���ʽ��Aת��ΪBCD�ķ�Ӧ���������Ƴ�AΪ
�����F�ķ���ʽC4H8O2��C��Dת��ΪF�ķ�Ӧ����������֪CΪ���ᣬDΪ�Ҵ���GΪ��ȩ��FΪ������������BCD�Ľṹ�����A�ķ���ʽ��Aת��ΪBCD�ķ�Ӧ���������Ƴ�AΪ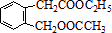 ��
��
��1��BΪ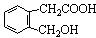 ������ʽΪC9H10O3��A�Ľṹ��ʽ��
������ʽΪC9H10O3��A�Ľṹ��ʽ��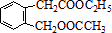 ����Ӧ��Ϊ����ˮ�ⷴӦ��
����Ӧ��Ϊ����ˮ�ⷴӦ��
�ʴ�Ϊ��C9H10O3��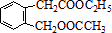 ��ˮ�⣻
��ˮ�⣻
��2����Ӧ��Ϊ������Ҵ���Ũ���������·���������Ӧ����Ӧ�ķ���ʽΪCH3COOH+C2H5OH
CH3COOC2H5+H2O��
�ʴ�Ϊ��CH3COOH+C2H5OH
CH3COOC2H5+H2O��
��3��GΪ��ȩ������-CHO������������Һ����������Ӧ����Ӧ�ķ���ʽΪCH3CHO+2Ag��NH3��2OH
CH3COONH4+2Ag��+3NH3+H2O��
�ʴ�Ϊ��CH3CHO+2Ag��NH3��2OH
CH3COONH4+2Ag��+3NH3+H2O��
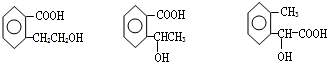
��4��BΪ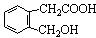 ����Ӧ��ͬ���칹���У��ٺ����ڶ�ȡ�������ṹ��
����Ӧ��ͬ���칹���У��ٺ����ڶ�ȡ�������ṹ��
����B����ͬ�����ţ�˵��ҳ����-COOH��-OH��
�۲���FeCl3��Һ������ɫ��Ӧ��˵���������ǻ�������ܵĽṹΪ ��������ͬ���칹�壬
��������ͬ���칹�壬
�ʴ�Ϊ��3��
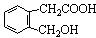 �����F�ķ���ʽC4H8O2��C��Dת��ΪF�ķ�Ӧ����������֪CΪ���ᣬDΪ�Ҵ���GΪ��ȩ��FΪ������������BCD�Ľṹ�����A�ķ���ʽ��Aת��ΪBCD�ķ�Ӧ���������Ƴ�AΪ
�����F�ķ���ʽC4H8O2��C��Dת��ΪF�ķ�Ӧ����������֪CΪ���ᣬDΪ�Ҵ���GΪ��ȩ��FΪ������������BCD�Ľṹ�����A�ķ���ʽ��Aת��ΪBCD�ķ�Ӧ���������Ƴ�AΪ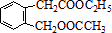 ��
����1��BΪ
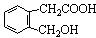 ������ʽΪC9H10O3��A�Ľṹ��ʽ��
������ʽΪC9H10O3��A�Ľṹ��ʽ��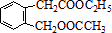 ����Ӧ��Ϊ����ˮ�ⷴӦ��
����Ӧ��Ϊ����ˮ�ⷴӦ���ʴ�Ϊ��C9H10O3��
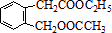 ��ˮ�⣻
��ˮ�⣻��2����Ӧ��Ϊ������Ҵ���Ũ���������·���������Ӧ����Ӧ�ķ���ʽΪCH3COOH+C2H5OH
| Ũ���� |
| �� |
�ʴ�Ϊ��CH3COOH+C2H5OH
| Ũ���� |
| �� |
��3��GΪ��ȩ������-CHO������������Һ����������Ӧ����Ӧ�ķ���ʽΪCH3CHO+2Ag��NH3��2OH
| �� |
�ʴ�Ϊ��CH3CHO+2Ag��NH3��2OH
| �� |
��4��BΪ
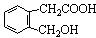 ����Ӧ��ͬ���칹���У��ٺ����ڶ�ȡ�������ṹ��
����Ӧ��ͬ���칹���У��ٺ����ڶ�ȡ�������ṹ�� ����B����ͬ�����ţ�˵��ҳ����-COOH��-OH��
�۲���FeCl3��Һ������ɫ��Ӧ��˵���������ǻ�������ܵĽṹΪ
 ��������ͬ���칹�壬
��������ͬ���칹�壬�ʴ�Ϊ��3��

���������⿼���л�����ƶϣ���Ŀ�Ѷ��еȣ�����ע�����B��F��Ϊ�ƶϵ�ͻ�ƿڣ�ע������л��ﷴӦ���������л�������ŵ����ʣ�Ϊ��������Ŀ�Ĺؼ���

��ϰ��ϵ�д�
 ������ҵ��ͬ����ϰ��ϵ�д�
������ҵ��ͬ����ϰ��ϵ�д� С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д�
�����Ŀ

 +2NaOH
+2NaOH +CH3COONa+CH3CH2OH
+CH3COONa+CH3CH2OH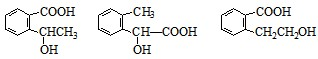 ������һ��
������һ�� ����CH2=CH2+H2O
����CH2=CH2+H2O ������ͼ�ش����⣺
������ͼ�ش����⣺