��Ŀ����
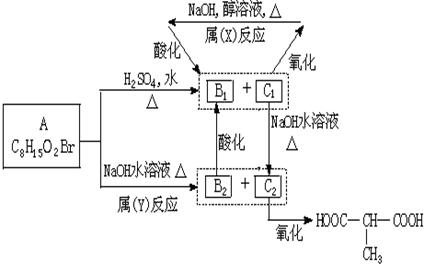
ij�л���A�ڲ�ͬ�����·�����ͼ��ʾ�ķ�Ӧ��C���ֱܷ�ת��ΪB��E��E�ܽ�һ����������
����ֻ��B����ʹ��ˮ��ɫ��������Na2CO3��Һ��Ӧ�ų�CO2��
��ش��������⣺
��1�����������������ͣ�B______������ţ���ͬ����E______��
��һԪ�� �ڶ�Ԫ�� �۷� ��ȩ �ݱ������� ����������
��2����Ӧ���ͣ���Ӧ��______����Ӧ��______��
��3������C�Ľṹ��ʽΪ______��
��4��F�ĺ˴Ź����������֮��Ϊ______��
��5��д����Ӧ�ڵĻ�ѧ����ʽ��______��
����ֻ��B����ʹ��ˮ��ɫ��������Na2CO3��Һ��Ӧ�ų�CO2��
��ش��������⣺
��1�����������������ͣ�B______������ţ���ͬ����E______��
��һԪ�� �ڶ�Ԫ�� �۷� ��ȩ �ݱ������� ����������
��2����Ӧ���ͣ���Ӧ��______����Ӧ��______��
��3������C�Ľṹ��ʽΪ______��
��4��F�ĺ˴Ź����������֮��Ϊ______��
��5��д����Ӧ�ڵĻ�ѧ����ʽ��______��

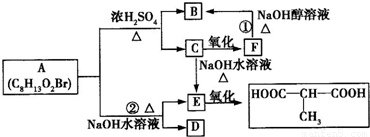
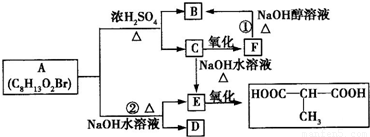
A�ķ���ʽC8H13O2Br�����ת����ϵ��֪��A��������������������������ˮ�⣬��A����1��������C��������F��F������ȥ��Ӧ����B����C�����ǻ�����ԭ�ӣ�B�����Ȼ�����C��F��B������ͬ��̼ԭ����Ŀ������4��̼ԭ�ӣ�Cˮ������E��E��������
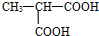
����
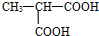
�Ľṹ��֪��CΪBrCH2CH��CH3��CH2OH����EΪHOCH2CH��CH3��CH2OH��˳�ƿɵã�FΪBrCH2CH��CH3��COOH��BΪCH2=C��CH3��COOH��A��ϡ�����з���ˮ�ⷴӦ����B��C����A�Ľṹ��ʽΪCH2=C��CH3��COOCH2CH��CH3��CH2Br��A����ˮ��õ�E��D����DΪCH2=CH��CH3��COONa��
��1��������������֪��BΪCH2=C��CH3��COOH������̼̼˫�������ᣬ���ڲ��������
EΪHOCH2CH��CH3��CH2OH�������к���2���ǻ������ڶ�Ԫ�����ʴ�Ϊ���ޣ��ڣ�
��2����ת����ϵ��֪����Ӧ��������ȥ��Ӧ����Ӧ������ˮ�ⷴӦ���ʴ�Ϊ����ȥ��Ӧ��ˮ�ⷴӦ��
��3��������������֪������C�Ľṹ��ʽΪ��BrCH2CH��CH3��CH2OH���ʴ�Ϊ��BrCH2CH��CH3��CH2OH��
��4��FΪBrCH2CH��CH3��COOH����������4�л�ѧ��ѧ������ͬ��Hԭ�ӣ���Ŀ�ֱ�Ϊ2��1��3��1����F�ĺ˴Ź����������֮��Ϊ2��1��3��1��
�ʴ�Ϊ��2��1��3��1��
��5����Ӧ�ڵĻ�ѧ����ʽΪCH2=C��CH3��COOCH2CH��CH3��CH2Br+2NaOH
CH2=CH��CH3��COONa+HOCH2CH��CH3��CH2OH+NaBr��
�ʴ�Ϊ��CH2=C��CH3��COOCH2CH��CH3��CH2Br+2NaOH
CH2=CH��CH3��COONa+HOCH2CH��CH3��CH2OH+NaBr��
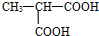
����
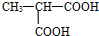
�Ľṹ��֪��CΪBrCH2CH��CH3��CH2OH����EΪHOCH2CH��CH3��CH2OH��˳�ƿɵã�FΪBrCH2CH��CH3��COOH��BΪCH2=C��CH3��COOH��A��ϡ�����з���ˮ�ⷴӦ����B��C����A�Ľṹ��ʽΪCH2=C��CH3��COOCH2CH��CH3��CH2Br��A����ˮ��õ�E��D����DΪCH2=CH��CH3��COONa��
��1��������������֪��BΪCH2=C��CH3��COOH������̼̼˫�������ᣬ���ڲ��������
EΪHOCH2CH��CH3��CH2OH�������к���2���ǻ������ڶ�Ԫ�����ʴ�Ϊ���ޣ��ڣ�
��2����ת����ϵ��֪����Ӧ��������ȥ��Ӧ����Ӧ������ˮ�ⷴӦ���ʴ�Ϊ����ȥ��Ӧ��ˮ�ⷴӦ��
��3��������������֪������C�Ľṹ��ʽΪ��BrCH2CH��CH3��CH2OH���ʴ�Ϊ��BrCH2CH��CH3��CH2OH��
��4��FΪBrCH2CH��CH3��COOH����������4�л�ѧ��ѧ������ͬ��Hԭ�ӣ���Ŀ�ֱ�Ϊ2��1��3��1����F�ĺ˴Ź����������֮��Ϊ2��1��3��1��
�ʴ�Ϊ��2��1��3��1��
��5����Ӧ�ڵĻ�ѧ����ʽΪCH2=C��CH3��COOCH2CH��CH3��CH2Br+2NaOH
| �� |
�ʴ�Ϊ��CH2=C��CH3��COOCH2CH��CH3��CH2Br+2NaOH
| �� |

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
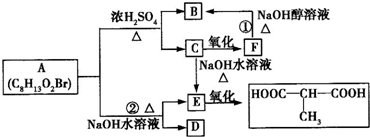 ij�л���A�ڲ�ͬ�����·�����ͼ��ʾ�ķ�Ӧ��C���ֱܷ�ת��ΪB��E��E�ܽ�һ����������
ij�л���A�ڲ�ͬ�����·�����ͼ��ʾ�ķ�Ӧ��C���ֱܷ�ת��ΪB��E��E�ܽ�һ����������