��Ŀ����
����ˮ�����ǹ�ϵ���ཡ������Ҫ���⡣
��1�����ҹ���һЩƶ��ɽ����ũ���������þ�ˮ�����������ˮ���ǣ������á�Ϊ�����ñ������ˮ�����ij��壬��Ὠ�����������ˮ��Ͷ�������е� ________������ţ���
A��NaCl B��Na2CO3 C��KAl(S O4)2��12H2O D��CuSO4
O4)2��12H2O D��CuSO4
��2��������������������ˮ���������ʣ�������������Ҫ����������ˮ�������˴����ᣬ�÷�Ӧ�����ӷ���ʽΪ��__________________________��
��3��д����ҵ����ȡƯ�۵Ļ�ѧ��Ӧ����ʽ��________________________��
��4��������Ҳ���������������������������ˮ�����������������Ч���Ұ�ȫ�����ã������ķ���ʽΪ_____________�������ʡ���ˮ�Լ�SO2��������Ư�������ߵ�Ư��ԭ���ֱ��ǣ�_________________��___________________��____________________��
��5��ClO2 ��һ�ֹ����͵�������������ȡ��Cl2��Ϊ��������ˮ������������ҵ��ClO2����NaClO3��Na2SO3��Һ��ϲ���H2SO4�ữ��Ӧ�Ƶã���Ӧ��NaClO3��Na2SO3�����ʵ���֮��Ϊ______________��
 ����������ϵ�д�
����������ϵ�д�

 ��ʱ,�ζ��ܼ�˴�������,�ζ�����ų����ݡ�
��ʱ,�ζ��ܼ�˴�������,�ζ�����ų����ݡ� �С�������ԭ���ǣ�____________�������ӷ���ʽ��ʾ����
�С�������ԭ���ǣ�____________�������ӷ���ʽ��ʾ���� ol��L-1
ol��L-1 CH3Cl+HCl
CH3Cl+HCl 2CH3CHO+2H2O
2CH3CHO+2H2O CH3COOC2H5+H2O
CH3COOC2H5+H2O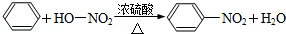
 eC��g��+ fD��g������Ӧ�����У���������������ʱ��C�İٷֺ�����C%�����¶ȣ�T����ѹǿ��P���Ĺ�ϵ��ͼ������������ȷ����
eC��g��+ fD��g������Ӧ�����У���������������ʱ��C�İٷֺ�����C%�����¶ȣ�T����ѹǿ��P���Ĺ�ϵ��ͼ������������ȷ����