��Ŀ����
��15�֣��Ҵ������ᶼ���л�������Ҫ�Ļ���ԭ�ϡ�
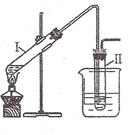
��1���������У��Ҵ�������ʹ����ͭ˿���ֺ��ȣ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��2����Ũ������£�������Ҵ�������������


ij��ѧ��ȤС���ͬѧ������װ�ý��и�������Ӧ��̽��ʵ�飺
��Ҫ����Թ��м�2mLŨ���ᡢ3mL�Ҵ���2mL���ᣬ���������� ��
��С�Թ���װ����ŨNa2CO3��Һ�������ܲ�����Һ������Ϊ�˷�ֹ ��
��������ʵIJ������ʣ�
�����ϱ�������ͬѧ�������ˮ���С�Թ��е�Na2CO3��Һ������Ϊ������˵�����ɣ� ���ܷ���С�Թ��е���������Ӧʹ�õ����������� ��
�ݷ���ʱ����������Ӧ�ô����� ����¿ڷš� ���Ͽڵ���������
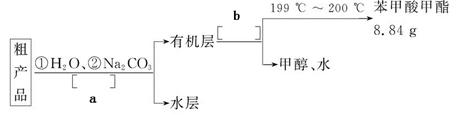
����ȤС�����������Ҵ����������������õ��������������������±���
��������X�ķ�Χ�� ��ʵ��a��ʵ��e̽����Ŀ���� ��
��1���������У��Ҵ�������ʹ����ͭ˿���ֺ��ȣ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��2����Ũ������£�������Ҵ�������������


ij��ѧ��ȤС���ͬѧ������װ�ý��и�������Ӧ��̽��ʵ�飺
��Ҫ����Թ��м�2mLŨ���ᡢ3mL�Ҵ���2mL���ᣬ���������� ��
��С�Թ���װ����ŨNa2CO3��Һ�������ܲ�����Һ������Ϊ�˷�ֹ ��
��������ʵIJ������ʣ�
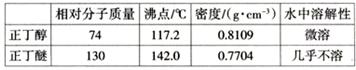
| | �Ҵ� | ���� | �������� |
| �е� | 78��0�� | 117��9�� | 77��5�� |
| ˮ���� | ���� | ���� | ���� |
�ݷ���ʱ����������Ӧ�ô����� ����¿ڷš� ���Ͽڵ���������
����ȤС�����������Ҵ����������������õ��������������������±���
| ʵ����� | �Ҵ���mL�� | ���ᣨmL�� | ����������mL�� |
| a | 2 | 2 | 1��33 |
| b | 3 | 2 | 1��57 |
| c | 4 | 2 | X |
| d | 5 | 2 | 1��76 |
| e | 2 | 3 | 1��55 |
��2CH3CH2OH + O2 2CH3CHO + 2H2O
2CH3CHO + 2H2O
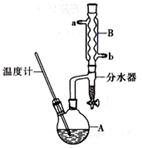
��2���ٴ��Թ��м���3mL�Ҵ���Ȼ������Թܱ���2mLŨ�����2mL���ᡣ�ڵ�����
�۲��ܣ������������Ҵ��������ˮ�Ļ����Һ���ܽ�ȱȽϴܷ�Һ©�� ���Ͽڵ���
��1��57-1��76mL�� ̽��������������������������Ӱ�졣
 2CH3CHO + 2H2O
2CH3CHO + 2H2O��2���ٴ��Թ��м���3mL�Ҵ���Ȼ������Թܱ���2mLŨ�����2mL���ᡣ�ڵ�����
�۲��ܣ������������Ҵ��������ˮ�Ļ����Һ���ܽ�ȱȽϴܷ�Һ©�� ���Ͽڵ���
��1��57-1��76mL�� ̽��������������������������Ӱ�졣
�����������1�������У��Ҵ�������ʹ����ͭ˿���ֺ��ȣ��÷�Ӧ�Ļ�ѧ����ʽΪ2CH3CH2OH + O2
 2CH3CHO + 2H2O; (2)��Ҫ����Թ��м�2mLŨ���ᡢ3mL�Ҵ���2mL���ᣬ����������������Թ��м���3mL�Ҵ���Ȼ������Թܱ���2mLŨ���ᣬ����Һ��ȴ���ټ���2mL���ᡣ��С�Թ���װ����ŨNa2CO3��Һ�������������ջӷ����Ҵ���������Ӧ�����������������ᣬ��������ζ�IJ��������������������ܽ�ȣ��Ա��ڻ����ķ����ᴿ�������ܲ�����Һ������Ϊ�˷�ֹ��������ķ������۲�����ˮ���С�Թ��е�Na2CO3��Һ������Ϊ�����������Ҵ��������ˮ�Ļ����Һ���ܽ�ȱȽϴܷ���С�Թ��л������ܵ�����Һ������ķ����Ƿ�Һ��ʹ�õ������Ƿ�Һ©�������ڷ���ʱ�����������������ܶȱ�ˮС�����ϲ㣬ʹ��Ӧ�ô������Ͽڵ��������ݱ������ݱ仯���ɿ�֪����������X�ķ�Χ��1��57-1��76mL��ʵ��a��ʵ��e���Ҵ���������ͬ��������������ͬ���Ҵ�������ʹ��̽����Ŀ����̽��������������������������Ӱ�졣
2CH3CHO + 2H2O; (2)��Ҫ����Թ��м�2mLŨ���ᡢ3mL�Ҵ���2mL���ᣬ����������������Թ��м���3mL�Ҵ���Ȼ������Թܱ���2mLŨ���ᣬ����Һ��ȴ���ټ���2mL���ᡣ��С�Թ���װ����ŨNa2CO3��Һ�������������ջӷ����Ҵ���������Ӧ�����������������ᣬ��������ζ�IJ��������������������ܽ�ȣ��Ա��ڻ����ķ����ᴿ�������ܲ�����Һ������Ϊ�˷�ֹ��������ķ������۲�����ˮ���С�Թ��е�Na2CO3��Һ������Ϊ�����������Ҵ��������ˮ�Ļ����Һ���ܽ�ȱȽϴܷ���С�Թ��л������ܵ�����Һ������ķ����Ƿ�Һ��ʹ�õ������Ƿ�Һ©�������ڷ���ʱ�����������������ܶȱ�ˮС�����ϲ㣬ʹ��Ӧ�ô������Ͽڵ��������ݱ������ݱ仯���ɿ�֪����������X�ķ�Χ��1��57-1��76mL��ʵ��a��ʵ��e���Ҵ���������ͬ��������������ͬ���Ҵ�������ʹ��̽����Ŀ����̽��������������������������Ӱ�졣
��ϰ��ϵ�д�
�����Ŀ
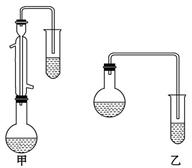
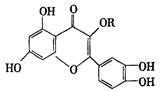
 ����Ľṹ��������
����Ľṹ��������