��Ŀ����
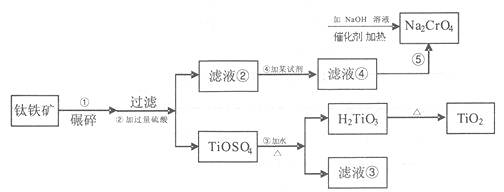
�ӻ�������������ȡ�����ƵĹ������£�
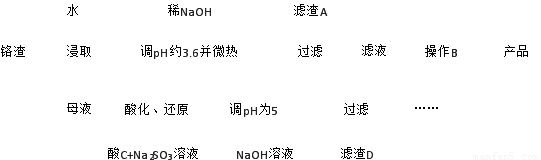
��֪������������Na2SO4������Cr2O72-��Fe3+����Fe3+��Cr3+��ȫ������c ��1.0��10-5 mol��L-1��ʱpH�ֱ�Ϊ3.6��5��
��1�����������ܼӿ췴Ӧ�����⣬ͬʱ������???????? ������AΪ ??????? ���ѧʽ����
��2�������ܽ�ȣ�S�����¶ȣ�T�����ߣ�����B����ѷ���Ϊ???????? ������ĸ��ţ�
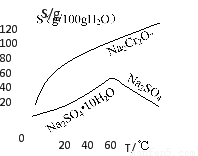
A������Ũ�������ȹ���
B������Ũ�������½ᾧ������
��3���ữ��Cr2O72���ɱ�SO32-��ԭ��Cr3+�����ӷ���ʽΪ��????? ????????????????? ����CΪ??????? ��Cr(OH)3���ܶȻ�����Ksp[Cr(OH)3]��???? ????????? ??? ��
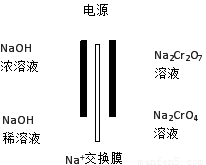
��4������2CrO42����2H+  Cr2O72����H2O���ͼʾװ�ã���Ϊ���Ե缫�����Na2CrO4��Һ��ȡNa2Cr2O7��ͼ���Ҳ�缫���ӵ�Դ��????? ������缫��ӦʽΪ?????????? ?????????? ��
Cr2O72����H2O���ͼʾװ�ã���Ϊ���Ե缫�����Na2CrO4��Һ��ȡNa2Cr2O7��ͼ���Ҳ�缫���ӵ�Դ��????? ������缫��ӦʽΪ?????????? ?????????? ��
��1���ٽ�Fe3��ˮ������Fe(OH)3����ȥ����2����Fe(OH)3����2����
��2��A��2����
��3��3SO32-��Cr2O72-��8H����2Cr3����3SO42-��4H2O����3������ѧʽ����ƽ����0����
H2SO4����2����1.0��10��32 mol4��L-4��2�����������1����
��4����������2����4OH��-4e����O2����2H2O��2���������������۷�1����
��������
�����������1�������ˮ�ⷴӦΪ���ȷ�Ӧ�����������ٽ�Fe3��ˮ������Fe(OH)3����ȥ��Fe3+ˮ������Fe(OH)3��������������AΪFe(OH)3��
��2�������ܽ�ȣ�S�����¶ȣ�T�����ߣ����Կ����¶Ƚϸ�ʱ�������¶ȵ����ߣ�Na2SO4���ܽ����С�����Բ�������Ũ�������ȹ����ķ�������A����ȷ��
��3���ữ��Cr2O72����SO32-����ΪSO42?���������ӷ���ʽΪ��3SO32-��Cr2O72-��8H����2Cr3����3SO42-��4H2O����Ϊ���ղ�ƷΪNa2SO4��Ϊ����������ʣ���CΪH2SO4��ǡ����ȫ����ʱ����Ũ��Ϊ1.0��10-5 mol��L-1��Cr3+��ȫ����ʱpHΪ5��c(OH?)= 1.0��10-9 mol��L-1������Cr(OH)3���ܶȻ�����Ksp[Cr(OH)3]��c(Cr3+)?c3(OH?)= 1.0��10-5 mol��L-1��(1.0��10-9 mol��L-1)3=1.0��10��32 mol4��L-4��
��4������ʾ��ͼ��ͼ���Ҳ�Na2CrO4ת��ΪNa2Cr2O7����ҪH+��˵���Ҳ�缫����H2O���������OH?�ŵ磬ʹH2O�ĵ���ƽ�������ƶ���H+���࣬�����Ҳ�缫���ӵ�Դ���������缫����ʽΪ��4OH��-4e����O2����2H2O
���㣺���⿼�黯ѧ�������̷���������ᾧ�����ӷ���ʽ����д���ܶȻ������ļ��㡢���ԭ����Ӧ�á�

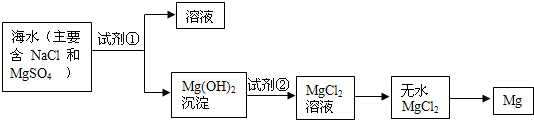
��10�֣�Ŀǰ������60%��þ�ǴӺ�ˮ����ȡ�ģ���ͼ��ij�����Ӻ�ˮ����ȡþ����Ҫ���衣ѧ�����������չ�������ۡ�ѧ�����������������������⣺ ��һ���ں�ˮ��þ�Ĺ��������ʵ�ֶ�þ���ӵĸ���
��һ���ں�ˮ��þ�Ĺ��������ʵ�ֶ�þ���ӵĸ���
������ѧ������Լ��Ĺ۵㡣 ѧ���Ĺ۵㣺ֱ������ˮ�м����������
ѧ���Ĺ۵㣺ֱ������ˮ�м���������� ѧ���ҵĹ۵㣺���¼���������ˮ���ټ����������
ѧ���ҵĹ۵㣺���¼���������ˮ���ټ���������� ѧ�����Ĺ۵㣺����ɹ�κ�Ŀ�±ˮ���ټ����������
ѧ�����Ĺ۵㣺����ɹ�κ�Ŀ�±ˮ���ټ���������� ͨ�������Ƚ�����Ϊѧ�� �Ĺ۵���ȷ����ѧ����ţ����������ɣ�
ͨ�������Ƚ�����Ϊѧ�� �Ĺ۵���ȷ����ѧ����ţ����������ɣ�  ��
��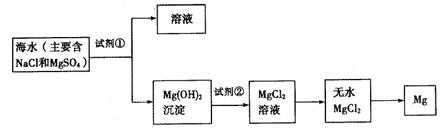


 �������ں�ˮ��þ�Ĺ��������ʵ�ֶ�þ���ӵķ��룿
�������ں�ˮ��þ�Ĺ��������ʵ�ֶ�þ���ӵķ��룿 ��1��Ϊ��ʹþ���ӳ�����������������õ��صı��ǣ���Ҫ�ɷ�Ϊ̼��ƣ���Դ������������Լ����� ���ѧʽ����
��1��Ϊ��ʹþ���ӳ�����������������õ��صı��ǣ���Ҫ�ɷ�Ϊ̼��ƣ���Դ������������Լ����� ���ѧʽ���� ��2�������Լ��ٺ��ܹ�����õ�Mg��OH��2�����ķ����� ��������ĸ��
��2�������Լ��ٺ��ܹ�����õ�Mg��OH��2�����ķ����� ��������ĸ��
| A������ | B������ | C����ȡ | D����Һ |
 ��3������������Լ����� ���ѧʽ����
��3������������Լ����� ���ѧʽ���� ��4��д������ˮMgCl2��ȡ����þ�Ļ�ѧ����ʽ
��4��д������ˮMgCl2��ȡ����þ�Ļ�ѧ����ʽ