��Ŀ����
��1��18��Ԫ���У���ϡ������Ԫ���⣬ѡ��ѧ������գ���1��ԭ�Ӱ뾶����Ԫ����______________��
��2��ԭ�Ӱ뾶��С��Ԫ����_____________�������______________��
��3�����ʵĻ�ԭ����ǿ��Ԫ����_______________��
��4�����ʵ���������ǿ��Ԫ����_______________��
��5������Ϊ��ɫ�����Ԫ����_________________��
��6����ˮ��Ӧ����ҵķǽ���������___________��
��7��ijԪ�ص�ԭ�Ӽ����γ������ӣ�Ҳ����ԭ������ʽ�γ������ӣ���������������Һ�з�Ӧ�����ӷ���ʽΪ____________��
��8��ijԪ��ԭ�ӵ�����������������֮��Ϊ2����ͼ������ﲻ��ȼ�գ�����������������������ܶԿ��������Ⱦ����Ԫ����________________��
�𰸣�
������
������
| ��1��Na����2��H��F����3��Na����4��F����5��F��Cl����6��F2����7��H++OH-=H2O����8��C
|

��ϰ��ϵ�д�
�����Ŀ

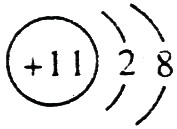 ��C��ԭ�ӽṹʾ��ͼ��
��C��ԭ�ӽṹʾ��ͼ��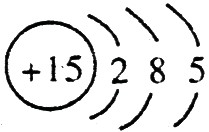 ��
��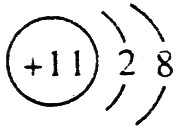 ����ԭ��������15����ԭ�ӽṹʾ��ͼ�ǣ�
����ԭ��������15����ԭ�ӽṹʾ��ͼ�ǣ�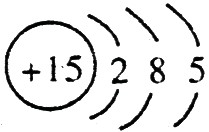 ��
�� ��1��
��1��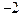 �������ӣ���д���Ľṹʾ��ͼ�� _____��
�������ӣ���д���Ľṹʾ��ͼ�� _____��