��Ŀ����
��֪2Fe3++2I-=2Fe2++I2��Br2+2Fe2+=2Br-+2Fe3+��������FeBr2��FeI2�Ļ��Һ��ͨ��һ�������������ٵμ�������KSCN��Һ����Һ��Ϊ��ɫ��������˵������ȷ����
A����I-��Fe2+��Br-��˳��ԭ������
B��ͨ��������ԭ��Һ��һ����Fe2+������
C��ԭ��Һ��Br-һ��������
D������ȷ��ͨ�����������Һ���Ƿ���Fe2+
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ


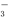 )��c(H2CO3
)��c(H2CO3 )��c(CO
)��c(CO )
) CO (g)+H2O(g) ��H
CO (g)+H2O(g) ��H
 ����
����
 ��C(OH-)��С
��C(OH-)��С