��Ŀ����
��12�֣�
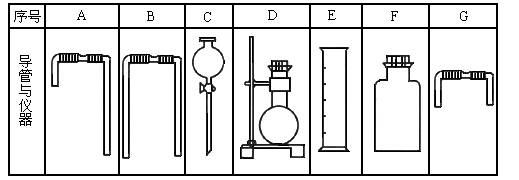
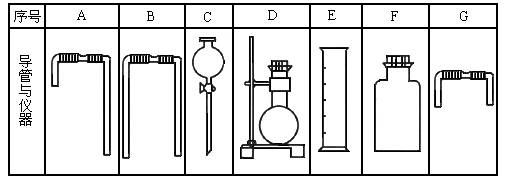
�����м���ʵ�飬�ɿ�����ȡʵ����������������壬�������������ʵ�顣��ʵ��װ������ͼ��ʾ��
��1����B������ʯ�ң�C��ʢ��AlCl3��Һ����A��Һ����μ���B�ڣ�ʵ��
�й۲쵽C��Һ�г��ְ�ɫ��������A��Һ������������������������ƣ�
C�з�����Ӧ�����ӷ���ʽΪ���������� ���������� ��
��2����Ҫ��O2��BΪ��ɫ���壬A�е��Լ��� ���ѧʽ����C��ʢ
��FeCl2��KSCN�Ļ��Һ��������Һ©���Ŀ��غ���C����Һ��Ϊ
Ѫ��ɫ��д��C �з���������ԭ��Ӧ�����ӷ���ʽ���������������������������������� ��
��3����֪������ǿ����KMnO4>Cl2>KIO3>I2����A��װ��Ũ���ᣬB��װ�й���KMnO4��C��ʢ��KI������Һ��C�е������������� ����������������������Ӧһ��ʱ�����C����Һ����ɫ��ȥ��������Ϊ�� ���������� ������������������������������
��12�֣�
��1��Ũ��ˮ��2�֣� Al3++3NH3��H2O= Al(OH)3��+3NH4+ ��2�֣�
��2�� H2O2��2�֣� 4Fe2++O2+4H+===4Fe3++2H2O��2�֣�
��3����Һ������2�֣�������Һ�еⵥ�ʱ���������������������ɫ��ȥ��2�֣�
����:��