��Ŀ����
���ֶ�����Ԫ��A��B��D��E��G��ԭ������������������A��Gͬ���壬 B��D��Eͬ���ڡ�A�ֱ���B��E��G�γ�ԭ�Ӹ�����Ϊ1:1�Ļ�����ס��ҡ����������� ���³�ѹ�·ֱ�Ϊ���塢Һ�塢���壬��ȼ��ʱ���������Ҳ���Ũ�ҵĺ��̣���Ϊ���Ӿ��塣
(1)D�Ļ�̬ԭ����______��δ�ɶԵ��ӣ��ĵ���ʽΪ____________
(2)����ˮ���ҷ�Ӧ����ǿ��X��A�ĵ��ʣ��仯ѧ����ʽΪ______��
(3)�ҵ�ˮ��Һ�������ԣ�����ˮ�еĵ��뷽��ʽΪ______��
(4)B��E����Ԫ�ذ�ԭ�Ӹ�����1:2�γɻ�����Y����X��Y�����ʵ���֮��Ϊ2:1 ��ȫ��Ӧ��������ҺŨ��Ϊ0.1 mol/L,���и�����Ũ�ȴӴ�С��˳������Ϊ______��
(5)��25��C��10lkPaʱ��16.0gҺ̬D2A4����������ȫȼ�շų�����312 kJ�������� ������Ⱦ���ʣ�����һ��Ϊ���ʣ���һ���������д���÷�Ӧ���Ȼ�ѧ����ʽ______��
 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�ʵ���У���ѡȡ�ķ���װ�����Ӧԭ������ȷ���� �� ��

ѡ�� | Ŀ �� | װ�� | ԭ �� |
A | ������������Ĵ��� | �� | �������Ӳ���ͨ����ֽ�����Ӽ�С���ӿ���ͨ����ֽ |
B | ���뱽�е��屽 | �� | ��(0.88 g/mL)���屽(1.5 g/mL)���ܶȲ�ͬ |
C | �����ᴿ | �٢� | NaCl��ˮ�е��ܽ�Ⱥܴ� |
D | ��ȥ�������еĻ�ϩ | �� | �������ķе�(161 ��)�뻷��ϩ�ķе�(83 ��)���ϴ� |
A. A B. B C. C D. D



 Na2S4O6+2NaI)
Na2S4O6+2NaI)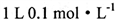 ��NH4NO3��Һ�е�ԭ����С��0.2NA
��NH4NO3��Һ�е�ԭ����С��0.2NA �ַ�Ӧʱת�Ƶĵ�����Ϊ1.2NA
�ַ�Ӧʱת�Ƶĵ�����Ϊ1.2NA Ca(OH)2(aq)
Ca(OH)2(aq)