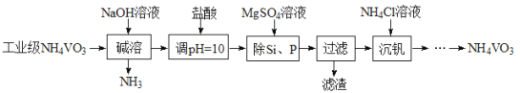
��Ŀ����
����Ŀ�������۵���ߵĽ���������Ҫ��ս�����ʡ���Ȼ��������Ҫ�����ں��ٿ��У�����Ҫ�ɷ��������̵������Σ�FeWO4��MnWO4������������Si��As�Ļ�����ɺ��ٿ�ұ���ٵĹ����������£�

��֪��������I����Ҫ�ɷ���Fe2O3��MnO2��
�����������У��ٵĻ��ϼ�ֻ�������һ�������ı䡣
������������������ˮ��
��1�������Σ�FeWO4��MnWO4������Ԫ�صĻ��ϼ�Ϊ____����д��MnWO4�����������·�����ֽⷴӦ����Fe2O3�Ļ�ѧ����ʽ__________��
��2�����������������������Һ�м������к���pH=10����Һ�е�����������ȷSiO32-��HAsO32-��HAsO42-�ȣ������������У�����H2O2ʱ������Ӧ�����ӷ���ʽΪ____������������Ҫ�ɷ���____��
��3����֪�������ƺ�����ƣ�CaWO4�������ܵ���ʣ����ߵ��ܽ�Ⱦ����¶����߶���С����ͼΪ��ͬ�¶���Ca��OH��2��CaWO4�ij����ܽ�ƽ�����ߣ���

��T1 _____T2���>����<����T1ʱKsp��CaWO4��=____��
������������Һ����ʯ����õ���������ƣ�������Ӧ�����ӷ���ʽΪ____��
���𰸡���+6 4FeWO4+O2+8NaOH![]() 2Fe2O3+4Na2WO4+4H2O
2Fe2O3+4Na2WO4+4H2O
��H2O2+HAsO32-�THAsO42-+H2O��MgSiO3 MgHAsO4
�Ǣ�< 1��10-10 ��WO42-+Ca��OH��2=CaWO4+2OH-
�������������̿�֪�������������������ơ�������Ӧ�����������������ƣ������̺��������Ʒ�Ӧ���������ƺ��������̣�ˮ��ʱ�����������������̲�����ˮ������������ˮ���ʹ��˺�õ�����Һ�������ƣ�����I����Ҫ�ɷ���Fe2O3��MnO2�������ƺ�Ũ���ᷴӦ��������������ƣ�����������⣬����+5�۵���Ϊ+6�ۣ������Ȼ�þ������������ˮ��MgSiO3��MgHAsO4�����ˣ���ҺΪ�����ƣ��ữ�����ȷֽ�����������ٺ�ˮ���û�ԭ����ԭ�������������٣��ݴ˷������
��1��������FeWO4Ϊ���������������Σ�FeWO4��MnWO4���������̵Ļ��ϼ۶�Ϊ+2�ۣ��������Σ�FeWO4��MnWO4������Ԫ�صĻ��ϼ�Ϊ+x���������������ϼ۴�����Ϊ�㣬��+2+x+��-2����4=0�����x=+6����������ͼ��֪�����������������ơ�������Ӧ�����������������ƣ���Ӧ�ķ���ʽΪ4FeWO4+O2+8NaOH![]() 2Fe2O3+4Na2WO4+4H2O����2���������Ϸ���������H2O2��Ŀ���ǽ�HAsO32-������HAsO42-�����ӷ���ʽΪH2O2+HAsO32-�THAsO42-+H2O����ҺI�д���SiO32-��HAsO32-��HAsO42-�����ӣ���������pHֵ�����Ȼ�þ��Mg2+����SiO32-��HAsO32-��HAsO42-�����ӣ�����������Ҫ�ɷ���MgSiO3��MgHAsO4
2Fe2O3+4Na2WO4+4H2O����2���������Ϸ���������H2O2��Ŀ���ǽ�HAsO32-������HAsO42-�����ӷ���ʽΪH2O2+HAsO32-�THAsO42-+H2O����ҺI�д���SiO32-��HAsO32-��HAsO42-�����ӣ���������pHֵ�����Ȼ�þ��Mg2+����SiO32-��HAsO32-��HAsO42-�����ӣ�����������Ҫ�ɷ���MgSiO3��MgHAsO4
����3��������ͼ���֪���������ƺ�������ڸ�����Ũ����ͬʱ��T1�¶���������Ũ�ȴ���T2��˵��T1ʱ���ܶȻ�����T2���ܶȻ�Խ�����ܽ��Խ������T1ʱ�ܽ�Ƚϴ����ڡ���֪�������ƺ�����ƣ�CaWO4�������ܵ���ʣ����ߵ��ܽ�Ⱦ����¶����߶���С������T1��T2��T1ʱKSP��CaWO4��=c��Ca2+��c��WO42-��=1��10-5��1��10-5=1��10-10��������������Һ����ʯ���飬�������ֽⷴӦ���������ƺ���������ӷ�Ӧ��������Ƴ�������Ӧ�����ӷ���ʽΪWO42-+Ca��OH��2=CaWO4+2OH-��

����Ŀ���ؽ���Ԫ�ظ��Ķ��Խϴ�����ˮ�辭������������ŷš�
��.ij��ҵ��ˮ����Ҫ����Cr3+��ͬʱ������������Fe3+��Al3+��Ca2+��Mg2+�ȣ������Խ�ǿ��Ϊ�������ã�ͨ�������������̴�����

ע�����������ӳ�����������������ʽ��ȫ����ʱ��Һ��pH���±�
�������� | Fe(OH)3 | Fe(OH)2 | Mg(OH)2 | Al(OH)3 | Cr(OH)3 |
pH | 3.7 | 9.6 | ll.l | 8 | 9(>9�ܽ�) |
(1)���������пɴ���H2O2������Լ���________(�����)��
A.Na2O2 B.HNO3 C.FeCl3 D.KMnO4
(2)����NaOH��Һ������ҺpH��8ʱ����ȥ��������________����֪�����ӽ�����֬��ԭ����Mn+��nNaR��MRn��nNa+���˲�������������ȥ������������__________��
A.Fe3+ B.Al3+ C.Ca2+ D.Mg2+
(3)��ԭ�����У�ÿ����172.8g Cr2O72-ת��4.8 mol e-���÷�Ӧ���ӷ���ʽΪ________________��
��.���������£����۸���Ҫ��Cr2O72-��ʽ���ڣ���ҵ�ϳ��õ�ⷨ������Cr2O72-�ķ�ˮ��ʵ����������ͼװ��ģ�����Cr2O72-�ķ�ˮ��������Ӧ��Fe-2e-==Fe2+��������Ӧʽ��2H++2e-==H2����

(1)���ʱ�ܷ���Cu�缫�����������ϵ�Fe�缫��________(��ܡ����ܡ�)��������______________��
(2)���ʱ����������Һ��Cr2O72-ת��ΪCr3+�����ӷ���ʽΪ______________��
(3)������Ӧ�õ��Ľ������������������ɳ�����ȫ�������ˮ�ĵ���ƽ��Ӱ��ǶȽ�����ԭ��_________________��
(4)����Һ�г�ʼ����0.1mol Cr2O72-�������ɵ�������ȫ��ת�����ɳ�����������________g��