��Ŀ����
��Դ�����ǵ�ǰ������������ٵ��������⣬ͬʱȫ�������ů����̬������������ͻ�����������ܡ�����ȼ�ϵ�ء���չ��̼�����ǻ�ѧ�����ߵ��о�����
I������ͨ��������ˮú���ķ����Ƶá�����CO(g)+H2O(g) CO2(g)+H2(g)
CO2(g)+H2(g)
��H<0����850��ʱ��ƽ�ⳣ��K=1��
��1���������¶ȵ�750��ʱ���ﵽƽ��ʱK 1������ڡ�����С�ڡ����ڡ���
��2��850��ʱ������һ�ݻ��ɱ���ܱ�������ͬʱ����1.0molCO��3molH2O��1.0molCO2
��x molH2����
�ٵ�x=5.0ʱ��������Ӧ�� �������Ӧ�����淴Ӧ����������С�
����Ҫʹ������Ӧ��ʼʱ������Ӧ������У���xӦ����������� ��
����850��ʱ������x=5.0��x=6.0���������ʵ�Ͷ�ϲ��䣬��������Ӧ�ﵽƽ���
���H2����������ֱ�Ϊa%��b%����a b������ڡ�����С�ڡ����ڡ���
II����֪4.6gҺ̬�Ҵ���ȫȼ�����ɶ�����̼��Һ̬ˮ�ų�����136kJ��molҺ̬ˮת
��Ϊ����ˮ����44kJ��������
��3����д���Ҵ�ȼ��������̬ˮ���Ȼ�ѧ����ʽ
��
��4����0.1mol�Ҵ�������������ȼ�գ��õ�������ȫ��ͨ�뵽100mL3mol/LNaOH��Һ�У�����HCO-3�ĵ��룬��������Һ��c(CO2-3) c(HCO-3)������ڡ�����С�ڡ����ڡ�����ԭ���� ����������������
I������ͨ��������ˮú���ķ����Ƶá�����CO(g)+H2O(g)
 CO2(g)+H2(g)
CO2(g)+H2(g) ��H<0����850��ʱ��ƽ�ⳣ��K=1��
��1���������¶ȵ�750��ʱ���ﵽƽ��ʱK 1������ڡ�����С�ڡ����ڡ���
��2��850��ʱ������һ�ݻ��ɱ���ܱ�������ͬʱ����1.0molCO��3molH2O��1.0molCO2
��x molH2����
�ٵ�x=5.0ʱ��������Ӧ�� �������Ӧ�����淴Ӧ����������С�
����Ҫʹ������Ӧ��ʼʱ������Ӧ������У���xӦ����������� ��
����850��ʱ������x=5.0��x=6.0���������ʵ�Ͷ�ϲ��䣬��������Ӧ�ﵽƽ���
���H2����������ֱ�Ϊa%��b%����a b������ڡ�����С�ڡ����ڡ���
II����֪4.6gҺ̬�Ҵ���ȫȼ�����ɶ�����̼��Һ̬ˮ�ų�����136kJ��molҺ̬ˮת
��Ϊ����ˮ����44kJ��������
��3����д���Ҵ�ȼ��������̬ˮ���Ȼ�ѧ����ʽ
��
��4����0.1mol�Ҵ�������������ȼ�գ��õ�������ȫ��ͨ�뵽100mL3mol/LNaOH��Һ�У�����HCO-3�ĵ��룬��������Һ��c(CO2-3) c(HCO-3)������ڡ�����С�ڡ����ڡ�����ԭ���� ����������������
��1�����ڣ�2�֣�
��2�����淴Ӧ��2�֣� ��x<3��2�֣� ��С�ڣ�2�֣�
��3��C2H5OH��l��+3C2��g��=2CO2��g��+3H2O��g�� ��H=-1228kJ/mol��2�֣�
��4��С�ڣ�2�֣� CO32- ˮ��̶ȴ���HCO3-ˮ��̶ȣ�2�֣�
��2�����淴Ӧ��2�֣� ��x<3��2�֣� ��С�ڣ�2�֣�
��3��C2H5OH��l��+3C2��g��=2CO2��g��+3H2O��g�� ��H=-1228kJ/mol��2�֣�
��4��С�ڣ�2�֣� CO32- ˮ��̶ȴ���HCO3-ˮ��̶ȣ�2�֣�
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
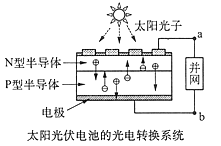
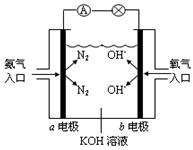
 ȼ��������̬ˮ���Ȼ�ѧ����ʽ��__________________________��
ȼ��������̬ˮ���Ȼ�ѧ����ʽ��__________________________�� �� ��ͨ���������õ����ݺͼ�������˵�����볣�����������������ʵij�ʼŨ�ȵĹ�ϵ �� ��
�� ��ͨ���������õ����ݺͼ�������˵�����볣�����������������ʵij�ʼŨ�ȵĹ�ϵ �� ��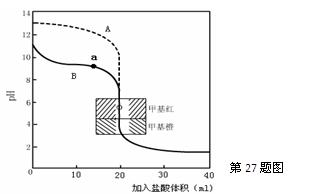
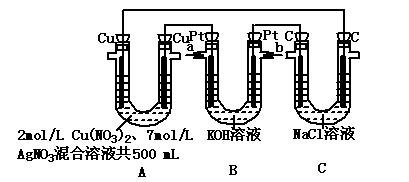

 2Ni(OH)2+Cd(OH)
2Ni(OH)2+Cd(OH) 2����˵������ȷ���ǣ��� ��
2����˵������ȷ���ǣ��� ��