��Ŀ����
��10�֣���ͼ���߿��е�װ�ÿ���������Ũ������ľ̿���ڼ��������·�Ӧ������������������д���пհס�����֪���������ܺ����Ը��������Һ����������ԭ��Ӧ����
��1��д��Ũ������ľ̿���ڼ��������·�Ӧ�Ļ�ѧ����ʽ ��
��2�������װ���Т١��ڡ�������������������˳���Ϊ�ڡ��١��ۣ����ܼ����������____ _��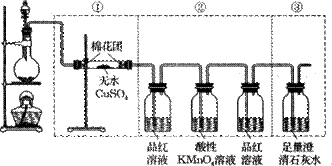
��3�����������������˳���Ϊ�١��ۡ��ڣ����ܼ����������_ _ _�� ����
��4��������ڢڲ�������������������KMnO4��Һ���װ��Ʒ����Һ��ϴ��ƿȥ��������������Ľ��ۻ���ʲôӰ�죿
��˵������ԭ�� ��
��1��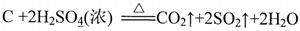
��2��H2O
��3��SO2 CO2
��4�������ж�������������ʯ��ˮ�����Ŷ�����̼�ļ��
����

��ϰ��ϵ�д�
 ��ѧ����ϵ�д�
��ѧ����ϵ�д� �ο�������ϵ�д�
�ο�������ϵ�д�
�����Ŀ



