��Ŀ����
(8��)��̼���ƺ����ᷴӦ�Ļ�ѧ����ʽΪ��2Na2CO4+4HCl=4NaCl+2CO2��+O2��+2H2O����Ʒ��̼������һ�㶼����Na2CO3��Ϊ�˲ⶨ���Ĵ��ȣ�ȡһ��������Ʒ�����ᷴӦ��ͨ��������������������������Լ������̼���Ƶĺ�����
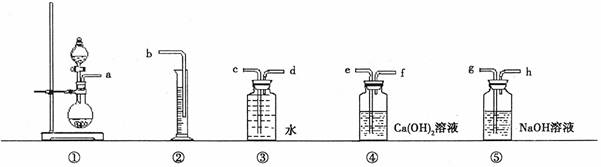
(1)������ͼ�ṩ������װ�ã���װһ�ײⶨ��Ʒ��̼���ƴ��ȵ�ʵ��װ�ã���Щװ�õ�����˳����(��ӿ���ĸ)�� ��
(2)װ�âܵ����ã�
��
(3)���ʵ��ʱ����ȡwg��Ʒ�������ᷴӦ���������������(��״��)ΪVmL����������Ʒ���ȵı���ʽΪ ��
(4)ij��ʵ�飬��ȡO.9g��Ʒ���вⶨ��ʵ��������50mL��100mL��150mL���ֹ�����Ͳ��Ӧѡ�ù��Ϊ ����Ͳ����ʵ�顣
(1)aghefdcb��2�֣�
��2��֤��CO2 �Ƿ��ѳ�����1�֣�
��3�� 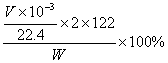 ��3�֣�
��3�֣�
��4��100mL ��2�֣�

��ϰ��ϵ�д�
�����Ŀ