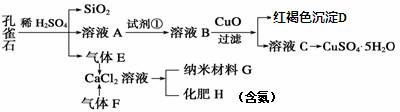
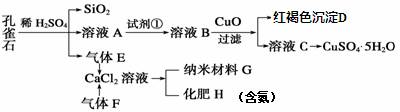
��Ŀ����
��
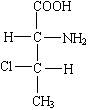
��������һ ���л����У���һ�����Ļ�ѧʽ��ͬ��ԭ�ӵ����ӷ�ʽ�ʹ���Ҳ��ͬ�����ṹʽ��ͬ������ԭ���ڿռ����еķ�ʽ�ϲ�ͬ���������ʵ��;���һ�������ֽṹ����Ȼ�����ֽṹ�dz����ƣ��������ص��������칹����ж�ӳ�칹����һ�Զ�ӳ�칹֮��Ĺ�ϵ�����˵����ֺ�����һ�������������ķ��ӽ����Է��ӡ�
�������϶� ���л�������У���ij��̼ԭ���������ĸ���ͬ��ԭ�ӻ�ԭ���ţ�����̼ԭ�Ӿͽ�������̼ԭ�ӻԳ�̼ԭ�ӣ���������̼ԭ�ӵķ���һ�������Է��ӣ����Է��ӾͿ��ܴ��ڶ�ӳ�칹���Իش��������⣺
��1����֪��3-��-2-������Ľṹ��ʽΪ�� һ��3-��-2-����������к���________������̼ԭ�ӡ�����һ�Զ�ӳ�칹���ü�ͶӰʽ��ʾΪ��
һ��3-��-2-����������к���________������̼ԭ�ӡ�����һ�Զ�ӳ�칹���ü�ͶӰʽ��ʾΪ��

����һ�Զ�ӳ�칹��ļ�ͶӰʽΪ��

________________________________________
��2����ѧʽΪ �ķ����������Է��ӣ�һ�������·������з�Ӧ�����ɵ��л�����Ȼ�����Է��ӵ���________��
�ķ����������Է��ӣ�һ�������·������з�Ӧ�����ɵ��л�����Ȼ�����Է��ӵ���________��
A�������ᷢ����������Ӧ
B����NaOH��Һ����
C���ڴ�����������H2����
D����������Һ����
��ij���л���CnHxOy����ȫȼ����ҪO2�����ʵ���Ϊ���л����n��������CO2��H2O�����ʵ���֮��Ϊ1��1��
��1�������л���ķ���ʽ��ͨʽΪ(CnHxOy�е�x��y����n��ʾ)________________��
��2����n=3��д�������������������ʵĽṹ��ʽ����дһ�֣���
�ٳ����ԣ����ܷ������Ϸ�Ӧ��________________��
�ڳ����ԣ����ܷ���ˮ�ⷴӦ��________________��
��3����һ�ַ��ϴ�ͨʽ���л����������һ����������ũ����Ľոѣ���Ҫ�ɷ�Ϊ��ά�أ���ȡ��д���÷�Ӧ�Ļ�ѧ����ʽ���л����ýṹ��ʽ��ʾ����________________��
������
��1��2�� ��2��D ��1��CnH2nOn ��2���� ��HCOOCH2CH2��OH��HO��CH2COOCH3 ������������� ��3�� ���������Ķ����⣬�ھ��м�ֵ����Ϣ���˽�����̼����ӳ�칹�ĺ����ǽ��������Ĺؼ��� ��1��������������࣬�����һ����ѧģ�ͣ��ǽ����Ҫ�ء�����CnHxOy��ȫȼ�����ɵ�CO2��H2O���ʵ���֮��Ϊ1��1���Ƶ�C��H=n��x=1��2�����Ƴ�x=2n�����CnHxOy��ȫȼ����O2n�������ȼ�շ�Ӧ��ѧʽ�����Ƴ�y=n������ͨʽCnH2nOn�� ��2�����n=3���Ƴ�CnH2nOn�Ļ�ѧʽC3H6O3��������ʣ������ṹ��ʽ�� ��3��ũ����ո���Ҫ�ɷ�����ά�أ�����ά�ؿ���ȡ�ķ���CnH2nOnͨʽ���л���Ӧ�������ǣ��ɴ˿�д����Ӧ�Ļ�ѧ����ʽ��
|

��Ŀ����ѧ�����ͨѶ����ͨ���ճ����������Ź㷺��Ӧ�á�
ǰ���õ�����Ni���ӣ�Cd����أ������ܷ�Ӧ���Ա�ʾΪ��
Cd��2NiO(OH)��2H2O�������� ![]() 2Ni(OH)2��Cd(OH)2
2Ni(OH)2��Cd(OH)2
��1����֪Ni(OH)2��Cd(OH)2��������ˮ���������ᣬ�ŵ����ʹ�õ�صĹ��̣�����Ǹ���ز�������Ĺ��̡�����˵������ȷ��������
�� ���Ϸ�Ӧ�������û���Ӧ�� �� ���Ϸ�Ӧ�Ǹ��ֽⷴӦ
�� ���ʱ��ѧ��ת��Ϊ���ܡ� �� �ŵ�ʱ��ѧ��ת��Ϊ����
A���٢ۡ� ������������ B���ڢܡ� ���������� C���٢ܡ� ���������� D���ڢ�
��2�����������ӵ���ѳ�Ϊ��Ҫ�Ļ�����Ⱦ������ϱ���һ�ڷ�������ؿ���ʹһƽ��������ĸ���ʧȥʹ�ü�ֵ��������������������Ⱦ��Ϊ���ء�������Ϊ���������� ����������������������������������������������������������������
��3����һ�ֳ��õĵ����﮵�أ�����һ�ּ����Ԫ�أ������ԭ������Ϊ7���������ı���������λ��������������ת���ĵ������ر����㷺Ӧ��������������һ��ʹ�õ�ʱ��ɳ���ʮ�ꡣ���ĸ����ý�����Ƴɣ�����ܷ�Ӧ�ɱ�ʾΪ��Li��MnO2��LiMnO2�Իش�﮵�ر������ر���ԭ��������������������������������������������������
﮵���еĵ������Һ���÷�ˮ�ܼ����ƣ�Ϊʲô���ֵ�ز���ʹ�õ���ʵ�ˮ��Һ�����û�ѧ����ʽ��ʾ��ԭ��������������������������������������������������������������
��4����ͼ�Ƿ������ֵ���е��ؽ�������ˮ����������һ��;����
������D���ؽ���Ũ������ �����ڡ����ڡ�С�ڣ�������A���ؽ���Ũ�ȣ�����ͨ��ʳ������������ ����������ġ�������;���⣬����Ⱦˮ���е��ؽ���������ֱ��ͨ������������ ��;����_________�������塣
��5����ͼ�������������ϵ�˵������ij�ͺŽ��ڵ��
ij�ͺŽ��ڵ�� | ij�ͺŹ������ |
| GNY 0.6��KR��AA�� |
�������ڵ�صĵ綯���������� ������������������ɷų������� ����ʱ�ĵ��������õ��ƽ����������Ϊ0.03����������ʹ�������� Сʱ��
 RECHARGEABLE
RECHARGEABLE