��Ŀ����
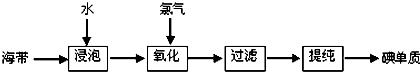
����һ���ս̰桶��ѧ1�����ޣ�����ҵ�ϴӺ���Ʒ���������л�ȡ�������ͼ���£�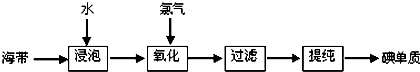
�ش�1������3������С�⣺
��1�������������̵ĵ�һ���ǡ����ݡ�������ʵ��Ŀ����
��2������������һ�����пɹ�ѡ�õ��Լ���Cl2��Br2��Ũ�����H2O2���μ�ϡ���ᣩ��������Ⱦ�Ƕȿ��ǣ�����Ϊѡ��ĺ����Լ���
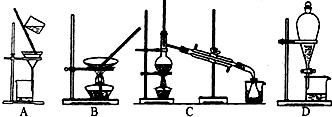
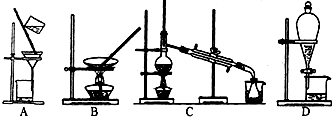
��3�����������������У����ᴿ��õ��ʵ⡱һ����Ϊ��������������ΪӦѡ���ʵ��װ�÷ֱ���
���϶����ҹ�ʵ�ʹ�ҵ���ú�����ȡ�⣬���õ������ӽ����������������£�
���ɺ��������ݡ�����Һ�������ϲ���Һ���ữ�����������ӽ�����֬���������������������ơ���Ʒ������������ȣ����ϲ���Һ������
�Իش�4������6������С�⣺
��4��ʵ�ʹ�ҵ�����У��ữ�������ķ������ȼ��������ữ��ʹpH���͵�2��Ȼ������������һ�����������ʹ��������������ҵͨ��������������ԭ���ǣ�
��5����������ʾ��Ϊ���ٻ�ѧ���ʶԻ�������Ⱦ����ҵ���Բ��õ绯ѧ���Ʊ�����
��6���������������������ӽ�����֬���ü�����֬������������������һ�ԭ��������������֬�����ĵ�Ԫ��״̬��
��������1�����ݲ����һ����֪�ò����ǵõ��������ӵ���Һ��
��2���������⽫�����������ɵⵥ�ʣ�����������ԭ��ˮ����������ȾҪ��
��3����������ȡ����ȡ�ⵥ�ʺ���������ȡ��ȡ���еĵ���н��
��4���������������ʽ��н�𣻴�����������Ԫ��Ϊ+1�ۣ�����ǿ�����ԣ�
��5�����ʱ��������������Ӧ������������ԭ��Ӧ�������ӱ�ɵⵥ�ʣ�����������Ӧ��
��6�������ҹ�ʵ�ʹ�ҵ���ú�����ȡ�������ͼ���н��
��2���������⽫�����������ɵⵥ�ʣ�����������ԭ��ˮ����������ȾҪ��
��3����������ȡ����ȡ�ⵥ�ʺ���������ȡ��ȡ���еĵ���н��
��4���������������ʽ��н�𣻴�����������Ԫ��Ϊ+1�ۣ�����ǿ�����ԣ�
��5�����ʱ��������������Ӧ������������ԭ��Ӧ�������ӱ�ɵⵥ�ʣ�����������Ӧ��
��6�������ҹ�ʵ�ʹ�ҵ���ú�����ȡ�������ͼ���н��
����⣺��1����������ˮ���������ݺͿ������������������������ǵ����ӣ����Ըò����ǴӺ����л�õ����ӣ�
�ʴ�Ϊ���Ӻ����л�õ����ӣ�
��2�������������������¿ɱ�MnO2����2I-+MnO2+4H+=Mn2++I2+2H2O�������������ǽ������ӶԻ�������Ⱦ��Cl2����2I-+Cl2�TI2+2Cl-������Cl-���Ի�������Ⱦ��˫��ˮ��H2O2�������������ӣ�2I-+H2O2+2H+�TI2+2H2O����������ԭ��ˮ����������ȾҪ��
�ʴ�Ϊ��H2O2��2I-+H2O2+2H+�TI2+2H2O��
��3���ᴿ��õ��ʵ⣬��������ȡ����ȡ��ˮ�еĵ⣬�÷�Һ©����ɵ���ȡװ�ã�ѡD������ȡ������ȡ�ⵥ�ʣ���������װ�ã���ѡC��
�ʴ�Ϊ��DC��
��4���������ж������壬�����Ի�����Ⱦ���Ҵ��������䲻���㣬������������Ԫ��Ϊ+1�ۣ�����ǿ�����ԣ��ɽ������������ɵ��ʵ⣬��������ԭ��-1�۵��ȣ�����ʽΪ2I-+ClO-+2H+�TI2+H2O+Cl-��
�ʴ�Ϊ�������Ի�����Ⱦ���Ҵ��������䲻���㣻2I-+ClO-+2H+�TI2+H2O+Cl-��
��5���绯ѧ���Ʊ����ʵ⣬�轫�����������ɵⵥ�ʣ�����������Ӧ�����ʱ��������������Ӧ����ӦΪ2I--2e-�TI2��
�ʴ�Ϊ��������2I--2e-�TI2��
��6�����ҹ�ʵ�ʹ�ҵ���ú�����ȡ�������ͼ��֪�����ü�����֬���������ڵ���������֮��˵����ʱ�������ǵⵥ�ʣ�������������̬��������������һ�ԭ��������˵���ⵥ�ʷ�����ԭ��Ӧ�����ɵ����ӣ���������������
�ʴ�Ϊ������̬��A��
�ʴ�Ϊ���Ӻ����л�õ����ӣ�
��2�������������������¿ɱ�MnO2����2I-+MnO2+4H+=Mn2++I2+2H2O�������������ǽ������ӶԻ�������Ⱦ��Cl2����2I-+Cl2�TI2+2Cl-������Cl-���Ի�������Ⱦ��˫��ˮ��H2O2�������������ӣ�2I-+H2O2+2H+�TI2+2H2O����������ԭ��ˮ����������ȾҪ��
�ʴ�Ϊ��H2O2��2I-+H2O2+2H+�TI2+2H2O��
��3���ᴿ��õ��ʵ⣬��������ȡ����ȡ��ˮ�еĵ⣬�÷�Һ©����ɵ���ȡװ�ã�ѡD������ȡ������ȡ�ⵥ�ʣ���������װ�ã���ѡC��
�ʴ�Ϊ��DC��
��4���������ж������壬�����Ի�����Ⱦ���Ҵ��������䲻���㣬������������Ԫ��Ϊ+1�ۣ�����ǿ�����ԣ��ɽ������������ɵ��ʵ⣬��������ԭ��-1�۵��ȣ�����ʽΪ2I-+ClO-+2H+�TI2+H2O+Cl-��
�ʴ�Ϊ�������Ի�����Ⱦ���Ҵ��������䲻���㣻2I-+ClO-+2H+�TI2+H2O+Cl-��
��5���绯ѧ���Ʊ����ʵ⣬�轫�����������ɵⵥ�ʣ�����������Ӧ�����ʱ��������������Ӧ����ӦΪ2I--2e-�TI2��
�ʴ�Ϊ��������2I--2e-�TI2��
��6�����ҹ�ʵ�ʹ�ҵ���ú�����ȡ�������ͼ��֪�����ü�����֬���������ڵ���������֮��˵����ʱ�������ǵⵥ�ʣ�������������̬��������������һ�ԭ��������˵���ⵥ�ʷ�����ԭ��Ӧ�����ɵ����ӣ���������������
�ʴ�Ϊ������̬��A��
������������Ҫ����Ӻ�������ȡ���ʵ�飬��ȷ���ʵķ��뷽�����ⵥ�ʵ������ǽ��Ĺؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ