��Ŀ����
������Ȼ�糣��������ˮ����������Ca3(PO4)2����ʽ���ڡ����ĵ��ʺͻ��������Ź㷺��Ӧ�á�

��1������P(s)��Cl2(g)������Ӧ����PCl3(g)��PCl5(g)����Ӧ���̺�������ϵ��ͼ��ʾ��ͼ�еġ�H��ʾ����1mol��������ݣ���
��ش����⣺
��PCl5�ֽ��PCl3��Cl2���Ȼ�ѧ����ʽ�� ��
��P��Cl2��������Ӧ����1 mol PCl5�ġ�H3�� ��
��2��PCl5�ֽ��PCl3��Cl2�ķ�Ӧ�ǿ��淴Ӧ��T��ʱ����2.0 L�����ܱ������г���1.0 mol PCl5������250 s�ﵽƽ�⡣��Ӧ�����вⶨ�IJ������ݼ��±���
�ٷ�Ӧ��50��150s �ڵ�ƽ������v(PCl3)�� ��
���Լ�����¶��·�Ӧ��ƽ�ⳣ����д��������̣�����2λ��Ч���֣�
��3��NaH2PO4��Na2HPO4��Na3PO4��ͨ��H3PO4��NaOH��Һ��Ӧ��ã��������ֵķֲ�����(ƽ��ʱij���ֵ�Ũ��ռ������Ũ��֮�͵ķ���)��pH �Ĺ�ϵ��ͼ��ʾ��

��Ϊ��ýϴ���Na2HPO4��pHӦ������ ��pH��6ʱ����Һ����Ҫ��������Ũ�ȴ�С��ϵΪ�� ��
��Na2HPO4��Һ�ʼ��ԣ�����������CaCl2��Һ����Һ�����ԣ���Һ�����Ե�ԭ���ǣ�������ƽ��Ƕȷ����� ��

��1������P(s)��Cl2(g)������Ӧ����PCl3(g)��PCl5(g)����Ӧ���̺�������ϵ��ͼ��ʾ��ͼ�еġ�H��ʾ����1mol��������ݣ���
��ش����⣺
��PCl5�ֽ��PCl3��Cl2���Ȼ�ѧ����ʽ�� ��
��P��Cl2��������Ӧ����1 mol PCl5�ġ�H3�� ��
��2��PCl5�ֽ��PCl3��Cl2�ķ�Ӧ�ǿ��淴Ӧ��T��ʱ����2.0 L�����ܱ������г���1.0 mol PCl5������250 s�ﵽƽ�⡣��Ӧ�����вⶨ�IJ������ݼ��±���
| t / s | 0 | 50 | 150 | 250 | 350 |
| n(PCl3) / mol | 0 | 0��16 | 0��19 | 0��20 | 0��20 |
�ٷ�Ӧ��50��150s �ڵ�ƽ������v(PCl3)�� ��
���Լ�����¶��·�Ӧ��ƽ�ⳣ����д��������̣�����2λ��Ч���֣�
��3��NaH2PO4��Na2HPO4��Na3PO4��ͨ��H3PO4��NaOH��Һ��Ӧ��ã��������ֵķֲ�����(ƽ��ʱij���ֵ�Ũ��ռ������Ũ��֮�͵ķ���)��pH �Ĺ�ϵ��ͼ��ʾ��

��Ϊ��ýϴ���Na2HPO4��pHӦ������ ��pH��6ʱ����Һ����Ҫ��������Ũ�ȴ�С��ϵΪ�� ��
��Na2HPO4��Һ�ʼ��ԣ�����������CaCl2��Һ����Һ�����ԣ���Һ�����Ե�ԭ���ǣ�������ƽ��Ƕȷ����� ��
��1����PCl5(g)=PCl3(g)+Cl2(g)��H����93kJ/mol��2�֣�����ʽ1�֣���H�ı�ʾ1�֣���ѧʽ��״̬����0�֣�+���ʱ���ֵ����λ��©�Ͽ�1�֣��������÷�����ʾ���ʱ���ƥ��Ҳ���֣�
�ڣ�399 kJ/mol��2�֣���λ��©��1�֣�
��2����1.5��10-4mol/(L��s) ��0.00015 mol/(L��s)��2�֣���λ��©��1�֣�
��2.5��10-2 mol/L��0.025mol/L
��3����9��10.5��2�֣����ڴ�����������ڵ�ijһ�㣩
c(H2PO4��)��c(HPO42��) ��2�֣�
��Na2HPO4��Һ�д��ڵ���ƽ�⣬HPO42��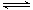 H++PO43����1�֣�������CaCl2��Һ��Ca2����PO43���������Ca3(PO4)2��������ʹNa2HPO4����ƽ�������ƶ���H+Ũ��������Һ�����ԣ�1�֣��������������֣�
H++PO43����1�֣�������CaCl2��Һ��Ca2����PO43���������Ca3(PO4)2��������ʹNa2HPO4����ƽ�������ƶ���H+Ũ��������Һ�����ԣ�1�֣��������������֣�
�ڣ�399 kJ/mol��2�֣���λ��©��1�֣�
��2����1.5��10-4mol/(L��s) ��0.00015 mol/(L��s)��2�֣���λ��©��1�֣�
��2.5��10-2 mol/L��0.025mol/L
��3����9��10.5��2�֣����ڴ�����������ڵ�ijһ�㣩
c(H2PO4��)��c(HPO42��) ��2�֣�
��Na2HPO4��Һ�д��ڵ���ƽ�⣬HPO42��
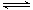 H++PO43����1�֣�������CaCl2��Һ��Ca2����PO43���������Ca3(PO4)2��������ʹNa2HPO4����ƽ�������ƶ���H+Ũ��������Һ�����ԣ�1�֣��������������֣�
H++PO43����1�֣�������CaCl2��Һ��Ca2����PO43���������Ca3(PO4)2��������ʹNa2HPO4����ƽ�������ƶ���H+Ũ��������Һ�����ԣ�1�֣��������������֣������������1����������ر�ע��ͼ������ת�����ʱ�������1mol��������ݣ����PCl5�ֽ��PCl3��Cl2�����ȷ�Ӧ�����Ȼ�ѧ����ʽΪ��PCl5(g)=PCl3(g)+Cl2(g)��H����93kJ/mol��
�ڼ���P��Cl2��������Ӧ����1 mol PCl5 ���ʱ䣬���ݸ�˹���ɷ�Ӧ���ʱ�������أ�ֻ�轫ͼ���������ֵ��ʱ���Ӽ��ɣ����ԡ�H3����399 kJ/mol��
��2���ټ��㷴Ӧ��50��150s �ڵ���PCl3��ʾ�ķ�Ӧ���ʣ�ֻ������ݴ��빫ʽ�������v(PCl3)����C/��t=(0��19-0��16)mol/(2L��100s)= 1.5��10-4mol/(L��s)��
�� PCl5(g) = PCl3(g)+Cl2(g)
��ʼŨ�ȣ�mol/L��
 =0.50 0 0
=0.50 0 0ת��Ũ�ȣ�mol/L�� 0.10
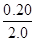 =0.10 0.10
=0.10 0.10 ƽ��Ũ�ȣ�mol/L�� 0.40 0.10 0.10 ��1�֣�
K��
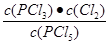 =
= 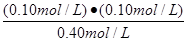 �� 2.5��10-2 mol/L��0.025mol/L
�� 2.5��10-2 mol/L��0.025mol/L��3������Ҫѧ�������ͼ����ͼ��ʾ����H3PO4�벻�ϼ����NaOH��Ӧ��������������������ʹ��ҺpHֵ��������ʱ����Һ�и��������ֵİٷֱȡ�ÿ������������һ����PH��Χ�ڶ��аٷֺ�������ͼ�С��������������߽��沿�ֱ�ʾ������2�ֺ�������ͬʱ���ڵ������
��Ҫ��ýд�����Na2HPO4����ͨ��ͼ���ҵ�PHֵ��9~10.5�ķ�Χ�ڣ�HPO42- �İٷֺ����ӽ��ٷְ٣����Կ��Ƶ�PHֻҪ�������Χ�ڣ������Ի�ýϴ�����Na2HPO4 ��
��Na2HPO4��������ʽ�Σ�HPO42-���ڵ����ˮ������ƽ�⣬������Һ�ʼ��ԣ����Կ���ˮ��ǿ�ڵ��롣���Ǹ�����Һ�����Ȼ��ƺ���Һ�����ԣ���˿����Ʋ������Ȼ���һ���ı��˵���ƽ�⣬ʹ֮���ϲ����������ӣ�ʹ��Һ�����ԡ����Խ�ϱ������ɡ�������Ȼ�糣��������ˮ����������Ca3(PO4)2����ʽ���ڡ������Խ���ƽ����ƶ�Ϊ��Na2HPO4��Һ�д��ڵ���ƽ�⣬HPO42��
 H++PO43��������CaCl2��Һ��Ca2����PO43���������Ca3(PO4)2��������ʹNa2HPO4����ƽ�������ƶ���H+Ũ��������Һ�����ԡ�
H++PO43��������CaCl2��Һ��Ca2����PO43���������Ca3(PO4)2��������ʹNa2HPO4����ƽ�������ƶ���H+Ũ��������Һ�����ԡ�
��ϰ��ϵ�д�
�����Ŀ
 FeO(s)+CO(g) ��H=akJ��mol-1,ƽ�ⳣ��ΪK;
FeO(s)+CO(g) ��H=akJ��mol-1,ƽ�ⳣ��ΪK; CO2(g) ��H=bkJ��mol-1;
CO2(g) ��H=bkJ��mol-1;
 2CO2(g)+N2(g)
2CO2(g)+N2(g)




 CH3OH(g)����ƽ��ʱ�����CO��H2��CH3OH�ֱ�Ϊ1 mol��1 mol��1 mol�����������Ϊ3L�����������м���ͨ��3 mol CO����ʱv������ v���棩��ѡ���>������<������=�������жϵ����� ��
CH3OH(g)����ƽ��ʱ�����CO��H2��CH3OH�ֱ�Ϊ1 mol��1 mol��1 mol�����������Ϊ3L�����������м���ͨ��3 mol CO����ʱv������ v���棩��ѡ���>������<������=�������жϵ����� �� CO(g)����H="-110.5" kJ��mol-1
CO(g)����H="-110.5" kJ��mol-1 ��
�� �� NO2�е������ļ���Ϊ466 kJ��mol��1��N2O4��N��N������Ϊ167 kJ��mol��1���������ļ���Ϊ438.5 kJ��mol��1��д��N2O4ת��ΪNO2���Ȼ�ѧ����ʽ ��
�� NO2�е������ļ���Ϊ466 kJ��mol��1��N2O4��N��N������Ϊ167 kJ��mol��1���������ļ���Ϊ438.5 kJ��mol��1��д��N2O4ת��ΪNO2���Ȼ�ѧ����ʽ ��  NiO(OH)��MH
NiO(OH)��MH

 CH3OH(g)��H2O(g)�� ��H����49.0 kJ��mol��1
CH3OH(g)��H2O(g)�� ��H����49.0 kJ��mol��1
 ��Ҫʹƽ������������ͬ��ֵ����������ȣ�����ʼʱά�ַ�Ӧ������У���c��ȡֵ��ΧΪ________��
��Ҫʹƽ������������ͬ��ֵ����������ȣ�����ʼʱά�ַ�Ӧ������У���c��ȡֵ��ΧΪ________��