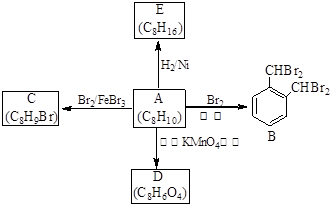��Ŀ����
����3���������A�� CH2=CH-CH3 B�� C��CH3CH2OH
C��CH3CH2OH
��1��д��������A��C�еĹ����ŵ����� �� ��
��2��A�ڴ������������¾ۺ����ɾۺ���ķ�Ӧ����ʽΪ ����Ӧ����Ϊ ��
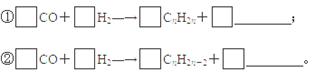
��3��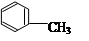 ��Ũ���������£���Ũ���Ṳ����100�淴Ӧ�Ļ�ѧ����ʽΪ�� ��
��Ũ���������£���Ũ���Ṳ����100�淴Ӧ�Ļ�ѧ����ʽΪ�� ��
��4��B���Ա����Ը�����������ɱ����ᣬд����������C������Ũ���Ṳ�ȷ���������Ӧ�Ļ�ѧ����ʽ�� ��
��1����̼̼˫�� �ǻ�
��2����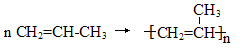 �Ӿ۷�Ӧ
�Ӿ۷�Ӧ
��3����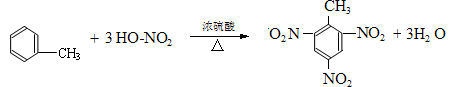
��4����C6H5COOH + CH3CH2OH  C6H5COOCH2CH3+H2O
C6H5COOCH2CH3+H2O
���������������1��A�к���̼̼˫����C�к����ǻ�����2��A����̼̼˫���ļӾ۷�Ӧ���ɾ۱�ϩ����3������������Ӧ���������������������ױ�����4������������Ӧ��
���㣺�����л���ѧ�л�ѧ������й����⡣

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д��ױ��Ƕ�ú���ۺ����õõ��IJ���֮һ����ṹ��ʽΪ  ���Իش��������⣺
���Իش��������⣺
(1)����ױ������ϵΪ_________��
| A��ͬ���칹�� | B��ͬλ�� |
| C��ͬ�������� | D��ͬϵ�� |
(3)�ױ������ϵ�һ�ȴ�����________�֡�
(4)��֪����
 �ṹ�����ʿɱ����Ը���������������ֱ��ͼױ��ķ�����________��
�ṹ�����ʿɱ����Ը���������������ֱ��ͼױ��ķ�����________�� ���ü�������������ȡ����Ӧ��ȡ����Ʒ����������ڹ�ҵ���ѳ�Ϊ��ʵ��ij��ѧ��ȤС��ͨ����ʵ������ģ���������̣�����Ƶ�ģ��װ�����£�
�������Ҫ��ش�
(1)Bװ�������ֹ��ܣ��ٿ��������ٶȣ��ھ��Ȼ�����壻��________��
(2)��V(Cl2)/V(CH4)��x��������������������Ȼ��⣬��xֵӦ________��
(3)Dװ�õ�ʯ���о��Ȼ���KI��ĩ����������_____________________________________��
(4)Eװ�õ�������___________________(����)��
| A���ռ����� | B���������� | C����ֹ���� | D�������Ȼ��� |
(6)Eװ�ó����������⣬�������л����E�з�����л������ѷ���Ϊ________����װ�û���ȱ�ݣ�ԭ����û�н���β����������β����Ҫ�ɷ�Ϊ________(����)��
a��CH4��b��CH3Cl��c��CH2Cl2��d��CHCl3��e��CCl4
Ϊ��������β���Դ�����ɵ���Ⱦ��Ŀǰ�г����Ƴ���ʹ���Ҵ����ͣ��������м��������Ҵ��������������������������
| A���Ҵ�������һ�ֻ����� | B������ʹ���Ҵ����Ϳ��Լ����к�������ŷ� |
| C���Ҵ����ȼ������CO2��H2O | D������ʳ���Ϳ��Ƶ��Ҵ� |