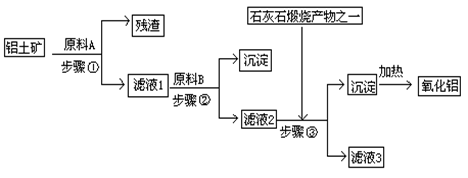
��Ŀ����
��ҵ���������ɷ�Ϊ������������������ȡ�����������£�

��ش��������⣺
��1�����������������õ��IJ���������
��2��д������ҺB����Al��OH��3�����ӷ���ʽ��
��3�������������漰������ԭ��Ӧ�Ļ�ѧ����ʽΪ
��4�����������У���NaOH��H2O����ѭ��ʹ���⣬������ѭ��ʹ�õ�������

��ش��������⣺
��1�����������������õ��IJ���������
�ձ�����ͨ©����������
�ձ�����ͨ©����������
����2��д������ҺB����Al��OH��3�����ӷ���ʽ��
2[Al��OH��4]-+CO2�T2Al��OH��3��+CO32-+H2O
2[Al��OH��4]-+CO2�T2Al��OH��3��+CO32-+H2O
����3�������������漰������ԭ��Ӧ�Ļ�ѧ����ʽΪ
2Al2O3�����ڣ�
4Al+3O2��
| ||
| ��� |
2Al2O3�����ڣ�
4Al+3O2��
��
| ||
| ��� |
��4�����������У���NaOH��H2O����ѭ��ʹ���⣬������ѭ��ʹ�õ�������
CaO��CO2
CaO��CO2
���ѧʽ�����ô˷���ȡ���ĸ���Ʒ��Fe2O3��O2
Fe2O3��O2
���ѧʽ������������1�����������������ǹ��ˣ��Դ��ж�ʹ��������
��2��Na[Al��OH��4]��Һ��ͨ��CO2����Al��OH��3������Na2CO3��
��3��ֻ�е������Al2O3�ķ�Ӧ����������ԭ��Ӧ��
��4�����ɵ�CaCO3���ȷֽ�õ�CaO��CO2���ɼ�CaO��CO2��ѭ��ʹ�ã����������õ�Fe2O3�͵������Al2O3�õ���O2Ϊ����Ʒ��
��2��Na[Al��OH��4]��Һ��ͨ��CO2����Al��OH��3������Na2CO3��
��3��ֻ�е������Al2O3�ķ�Ӧ����������ԭ��Ӧ��
��4�����ɵ�CaCO3���ȷֽ�õ�CaO��CO2���ɼ�CaO��CO2��ѭ��ʹ�ã����������õ�Fe2O3�͵������Al2O3�õ���O2Ϊ����Ʒ��
����⣺��1�����������������ǹ��ˣ�������Ҫ�ձ���©�������������ʴ�Ϊ���ձ�����ͨ©������������
��2��Na[Al��OH��4]��Һ��ͨ��CO2����Al��OH��3������Na2CO3�������ӷ���ʽΪ2[Al��OH��4]-+CO2�T2Al��OH��3��+CO32-+H2O��
�ʴ�Ϊ��2[Al��OH��4]-+CO2�T2Al��OH��3��+CO32-+H2O��
��3��ֻ�е������Al2O3�ķ�Ӧ����������ԭ��Ӧ����Ӧ�ķ���ʽΪ2Al2O3�����ڣ�
4Al+3O2�����ʴ�Ϊ��2Al2O3�����ڣ�
4Al+3O2����
��4�����ɵ�CaCO3���ȷֽ�õ�CaO��CO2���ɼ�CaO��CO2��ѭ��ʹ�ã����������õ�Fe2O3�͵������Al2O3�õ���O2Ϊ����Ʒ��
�ʴ�Ϊ��CaO��CO2��Fe2O3��O2��
��2��Na[Al��OH��4]��Һ��ͨ��CO2����Al��OH��3������Na2CO3�������ӷ���ʽΪ2[Al��OH��4]-+CO2�T2Al��OH��3��+CO32-+H2O��
�ʴ�Ϊ��2[Al��OH��4]-+CO2�T2Al��OH��3��+CO32-+H2O��
��3��ֻ�е������Al2O3�ķ�Ӧ����������ԭ��Ӧ����Ӧ�ķ���ʽΪ2Al2O3�����ڣ�
| ||
| ��� |
| ||
| ��� |
��4�����ɵ�CaCO3���ȷֽ�õ�CaO��CO2���ɼ�CaO��CO2��ѭ��ʹ�ã����������õ�Fe2O3�͵������Al2O3�õ���O2Ϊ����Ʒ��
�ʴ�Ϊ��CaO��CO2��Fe2O3��O2��
���������⿼�����ʵ��Ʊ��������ڽ�����ұ����ע��ѧ����˫�������Ŀ��飬ע��������ʵ����ʣ��ѶȲ���

��ϰ��ϵ�д�
�����Ŀ