��Ŀ����
̫���ܵ�ذ����̫��ʱ��һ���ֵ���ֱ�ӹ������칬һ�š���һ���ֵ������ڵ���������̫��ʱʹ�á����칬һ�š�ʹ�õ��������أ��������Һ�Լ��ԡ��䷴Ӧ����ʽΪ�� LaNi5+Ni(OH)2 LaNi5H+NiOOH�������й�˵������ȷ����
LaNi5H+NiOOH�������й�˵������ȷ����
A. �ŵ�ʱ������ԭ B. �ŵ�ʱ����LaNi5H+OH����e����LaNi5+H2O
C. ���ʱOH���������ƶ� D. ���ʱÿ����lmol Ni(OH)2ת��lmol����
��ϰ��ϵ�д�
�����Ŀ
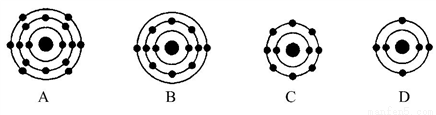
�������������ʵ�Ľ�����ȷ����
ѡ�� | �������ʵ | ���� |
A | ���ȵĴ�����Һϴȥ���� | Na2CO3��ֱ�������۷�Ӧ |
B | Ư���ڿ����о��ñ��� | Ư���е�CaCl2������е�CO2��Ӧ����CaCO3 |
C | ��Mg��OH��2����Һ�еμ�CuSO4��Һ��������ɫ���� | Cu��OH��2���ܶȻ���Mg��OH��2��С��Mg��OH��2ת��ΪCu��OH��2 |
D | ʩ��ʱ����ľ�ң���Ч�ɷ�ΪK2CO3��������NH4Cl���ʹ�� | K2CO3��NH4Cl��Ӧ����ʧ�ط� |
A. A B. B C. C D. D



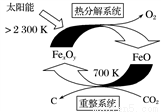
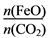 ��6����FexOy�Ļ�ѧʽΪ________��
��6����FexOy�Ļ�ѧʽΪ________�� ֵ��С
ֵ��С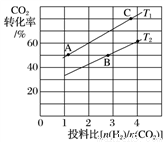
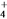 )________(�>������<������)c(HCO
)________(�>������<������)c(HCO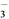 )����ӦNH
)����ӦNH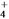 ��HCO
��HCO