��Ŀ����
��ĩ״����A���ɵ����ʵ�����MgO��Fe2O3��ɵĻ���
��������ʵ�飺������13�֣�
��. ȡ����A�������ȷ�Ӧ���������е���B���ɣ�
��. ��ȡ20gAȫ������0.15L6.0mol/L�����У�����ҺC��
��. �����еõ��ĵ���B����ҺC��Ӧ���ų�1.12L����������塣ͬʱ������ҺD���������й�������B��
��. ��KSCN��Һ���ʱ����ҺD����ɫ��
��. �ٲ����еĵ����� �ߣߣߣߣߣߡ��÷�Ӧ�Ļ�ѧ����ʽΪ
��. �����������ĸ���Ӧ�Ļ�ѧ����ʽ�� �ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��. �����������ĸ���Ӧ�����ӷ���ʽ�� �ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��. ����ҺD���������Ϊ0.15L�������Һ��c(Mg2+)Ϊ �ߣߣߣߣߣߣߣߣ�
��������ʵ�飺������13�֣�
��. ȡ����A�������ȷ�Ӧ���������е���B���ɣ�
��. ��ȡ20gAȫ������0.15L6.0mol/L�����У�����ҺC��
��. �����еõ��ĵ���B����ҺC��Ӧ���ų�1.12L����������塣ͬʱ������ҺD���������й�������B��
��. ��KSCN��Һ���ʱ����ҺD����ɫ��
��. �ٲ����еĵ����� �ߣߣߣߣߣߡ��÷�Ӧ�Ļ�ѧ����ʽΪ
��. �����������ĸ���Ӧ�Ļ�ѧ����ʽ�� �ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��. �����������ĸ���Ӧ�����ӷ���ʽ�� �ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��. ����ҺD���������Ϊ0.15L�������Һ��c(Mg2+)Ϊ �ߣߣߣߣߣߣߣߣ�
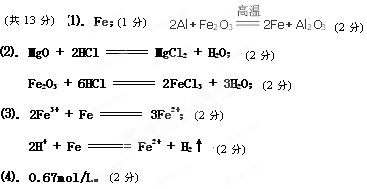
��. �ٲ����еĵ�����Fe,2Al��Fe2O3
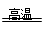 2Fe��Al2O3
2Fe��Al2O3��. ����ȡ20gA�к���MgO��Fe2O3,�����ķ�Ӧ��;MgO��2HCl=MgCl2��H2O,Fe2O3��6HCl=2FeCl3��3H2O;
�ǽ����еõ��ĵ���BΪFe,����ҺC��FeCl3��������HCl��Ӧ��2Fe3����Fe=3Fe2�� ,2H����Fe=Fe2����H2��
����MgOΪxmol,����40g/molx+160g/molx="20g,x=0.1mol," c(Mg2+)=0.1mol/0.15L=0.67mol/L

��ϰ��ϵ�д�
�����Ŀ
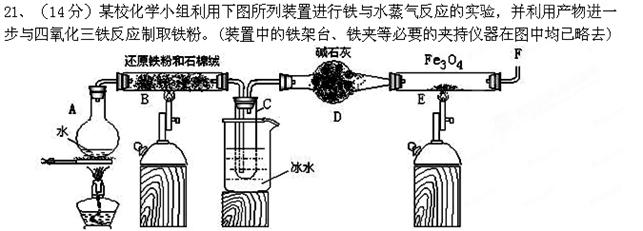
 ��KSCN��Һ����Һ��ɫ�����Ա仯���Խ���ԭ�� ��
��KSCN��Һ����Һ��ɫ�����Ա仯���Խ���ԭ�� ��
 _______������ĸ����
_______������ĸ���� �м������ϡ���ᣬ�õ�0.448 L(��״����)һ����ɫ����.д����Ӧ�����ӷ���ʽ�� ��������Һ��c(Cu2��)����������mol/L.
�м������ϡ���ᣬ�õ�0.448 L(��״����)һ����ɫ����.д����Ӧ�����ӷ���ʽ�� ��������Һ��c(Cu2��)����������mol/L.