��Ŀ����
��8�֣�AΪ����BΪ���ĺ���������ɵ����ʵ�����A��B��ɵĻ����0.05mol��0.125mol����������ȫȼ�գ�����0.1mol CO2��0.1mol H2O����ͨ������ش�
(1)��A��B��ɻ�����ƽ������ʽΪ___________
(2) ��ȡһ������A��B��ȫȼ�գ��������������ʵ����Ȼ�ϣ������ʵ���֮��һ��������������һ������A��B�ķ���ʽ�ֱ���___________�� ____________
�������ɵĶ�����̼��ˮ�����ʵ���һ������A��B�ķ���ʽ�ֱ��� �� (3) ��ȡa mol ��������Ȼ�ϵ�A��B�Ļ����ڹ�������������ȫȼ�ա�
����������Ϊ��ֵ���������Ϊ_____________mol(�ú�a�Ĵ���ʽ��ʾ����ͬ)
�������ɵ�CO2����Ϊ��ֵ����������ˮ��������ΧΪ__________________________��
���𰸡�
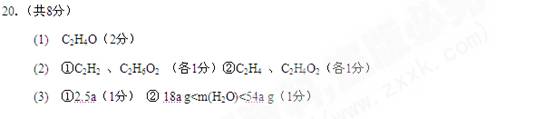
����������

��ϰ��ϵ�д�
�����Ŀ
