ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ―«œθΥᬻ(C1NO) «”–ΜζΚœ≥…÷–ΒΡ÷Ί“Σ ‘ΦΝΓΘΩ…”…NO”κCl2‘ΎΆ®≥ΘΧθΦΰœ¬Ζ¥”ΠΒΟΒΫΘ§Μ·―ßΖΫ≥Χ ΫΈΣ2NO(g)+C12(g)![]() 2C1NO(g)Θ§
2C1NO(g)Θ§
Θ®1Θ©ΒΣ―θΜ·Έο”κ–ϋΗΓ‘Ύ¥σΤχ÷–ΒΡΚΘ―ΈΝΘΉ”œύΜΞΉς”Ο ±Μα…ζ≥…―«œθΥᬻȧ…φΦΑ»γœ¬Ζ¥”ΠΘΚ
ΔΌ 2NO2(g)+NaC1(s)![]() NaNO3(s)+ClNO(g) K1
NaNO3(s)+ClNO(g) K1
ΔΎ 4NO2(g)+2NaC1(s)![]() 2NaNO3(s)+2NO(g)+Cl2(g) K2
2NaNO3(s)+2NO(g)+Cl2(g) K2
Δέ 2NO(g)+C12(g)![]() 2C1NO(g) K3
2C1NO(g) K3
‘ρK1Θ§K2Θ§K3÷°ΦδΒΡΙΊœΒΈΣK3=______________ΓΘ
Θ®2Θ©“―÷ΣΦΗ÷÷Μ·―ßΦϋΒΡΦϋΡή ΐΨί»γœ¬±μ(―«œθΥᬻΒΡΫαΙΙΈΣCl-N=O):

‘ρ2NO(g)+C12(g)![]() 2C1NO(g)Ζ¥”ΠΒΡΓςHΚΆaΒΡΙΊœΒΈΣΓςH=________kJ/molΓΘ
2C1NO(g)Ζ¥”ΠΒΡΓςHΚΆaΒΡΙΊœΒΈΣΓςH=________kJ/molΓΘ
Θ®3Θ©‘Ύ1LΒΡΚψ»ίΟή±’»ίΤς÷–≥δ»κ2molNO(g)ΚΆ1molC12(g)Θ§‘Ύ≤ΜΆ§Έ¬Ε»œ¬≤βΒΟc(C1NO)”κ ±ΦδΒΡΙΊœΒ»γΆΦA:
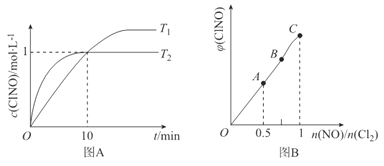
ΔΌ ”…ΆΦAΩ…≈–ΕœT1 ________T2Θ§ΗΟΖ¥”ΠΒΡΓςH________0 (ΧνΓΑ>Γ±ΓΑ<Γ±ΜρΓΑ=Γ±)ΓΘ
ΔΎ Ζ¥”ΠΩΣ ΦΒΫ10min ±NOΒΡΤΫΨυΖ¥”ΠΥΌ¬ v(NO)=____________mol/(LΓΛmin)ΓΘ
Δέ T2 ±ΗΟΖ¥”ΠΒΡΤΫΚβ≥Θ ΐK=____________ΓΘ
Θ®4Θ© “ΜΕ®ΧθΦΰœ¬‘ΎΚψΈ¬Κψ»ίΒΡΟή±’»ίΤς÷–Α¥“ΜΕ®±»άΐ≥δ»κNO(g)ΚΆCl2(g)Θ§ΤΫΚβ ±ClNOΒΡΧεΜΐΖ÷ ΐΥφn(NO)/n(C12)ΒΡ±δΜ·ΆΦœσ»γΆΦBΘ§‘ρAΓΔBΓΔC»ΐΉ¥Χ§÷–Θ§NOΒΡΉΣΜ·¬ Ήν¥σΒΡ «________ΒψΓΘ
ΓΨ¥πΑΗΓΩ K12/K2 289-2a < < 0.1 2 A
ΓΨΫβΈωΓΩ ‘ΧβΖ÷ΈωΘΚΘ®1Θ©“―÷ΣΘΚΔΌ2NO2(g)+NaCl(s)![]() NaNO3(s)+ClNO(g)
NaNO3(s)+ClNO(g)
ΔΎ 4NO2(g)+2NaCl(s)![]() 2NaNO3(s)+2NO(g)+Cl2(g)
2NaNO3(s)+2NO(g)+Cl2(g)
ΗυΨίΗ«ΥΙΕ®¬…Ω…÷ΣΫΪΔΌΓΝ2-ΔΎΩ…ΒΟΘΚ2NO(g)+Cl2(g)![]() 2ClNO(g)Θ§“ρ¥ΥΗΟΖ¥”ΠΒΡΤΫΚβ≥Θ ΐK3ΘΫK12/K2ΓΘ
2ClNO(g)Θ§“ρ¥ΥΗΟΖ¥”ΠΒΡΤΫΚβ≥Θ ΐK3ΘΫK12/K2ΓΘ
Θ®2Θ©2NO(g)+C12(g)![]() 2C1NO(g)Ζ¥”ΠΒΡΓςH=Ζ¥”ΠΈοΒΡΦϋΡή÷°ΚΆ-…ζ≥…ΈοΒΡΦϋΡή÷°ΚΆ=(2ΓΝ630+243) kJ/mol-(2a+2ΓΝ607) kJ/molΘΫ(289-2a)kJ/molΓΘ
2C1NO(g)Ζ¥”ΠΒΡΓςH=Ζ¥”ΠΈοΒΡΦϋΡή÷°ΚΆ-…ζ≥…ΈοΒΡΦϋΡή÷°ΚΆ=(2ΓΝ630+243) kJ/mol-(2a+2ΓΝ607) kJ/molΘΫ(289-2a)kJ/molΓΘ
Θ®3Θ©ΔΌ ΗυΨίΆΦœώΩ…÷ΣT2ΘΨT1Θ§ΫΒΒΆΈ¬Ε»c(C1NO)‘ω¥σΘ§ΥΒΟςΤΫΚβœρ’ΐΖ¥”ΠΖΫœρ“ΤΕ·Θ§ΥΒΟς’ΐΖ¥”Π «Ζ≈»»Ζ¥”ΠΘ§ΓςHΘΦ0ΓΘ
ΔΎ Ζ¥”ΠΩΣ ΦΒΫ10min ±Θ§c(C1NO)=1mol/LΘ§‘ρv(C1NO)= 1mol/LΓ¬10min=0.1mol/(LΓΛmin)Θ§‘ρNOΒΡΤΫΨυΖ¥”ΠΥΌ¬ v(NO)=v(C1NO)=0.1mol/(LΓΛmin)
Δέ 2NO(g)+Cl2(g)![]() 2ClNO(g)
2ClNO(g)
Τπ Φ(mol/L) 2 1 0
Ζ¥”Π(mol/L) 1 0.5 1
ΤΫΚβ(mol/L) 1 0.5 1
Υυ“‘T2 ±ΗΟΖ¥”ΠΒΡΤΫΚβ≥Θ ΐK=![]() =12/12ΓΝ0.5=2ΓΘ
=12/12ΓΝ0.5=2ΓΘ
Θ®4Θ©n(NO)/n(C12)ΒΡ±»÷Β‘Ϋ–ΓΘ§ΥΒΟς»τn(NO)≤Μ±δΘ§n(C12) ‘Ϋ¥σΘ§Υυ“‘NOΒΡΉΣΜ·¬ ‘Ϋ¥σΘ§NOΒΡΉΣΜ·¬ Ήν¥σΒΡ «AΒψΓΘ

ΓΨΧβΡΩΓΩΩΊ÷Τ±δΝΩ «ΩΤ―ß―–ΨΩΒΡ÷Ί“ΣΖΫΖ®ΓΘœύΆ§÷ ΝΩΒΡ¬Ν”κΉψΝΩ1 molΓΛL-1―ΈΥαΖ÷±π‘Ύœ¬Ν–ΧθΦΰœ¬ΖΔ…ζΖ¥”ΠΘ§ΩΣ ΦΫΉΕΈΜ·―ßΖ¥”ΠΥΌ¬ Ήν¥σΒΡ «(ΓΓΓΓ)
―Γœν | ¬ΝΒΡΉ¥Χ§ | Β―ιΈ¬Ε»/Γφ |
A | Τ§Ή¥ | 20 |
B | Τ§Ή¥ | 30 |
C | ΖέΡ© | 20 |
D | ΖέΡ© | 30 |
A.AB.BC.CD.D