��Ŀ����
(5��)��һƿ�������Һ�����п��ܺ���H+��NH4+��Mg2+��Ba2+��Al3+��I-��NO3-��CO32-��SO42-��AlO2-��ȡ����Һ��������ʵ�飺
(1)ȡpH��ֽ���飬��Һ��ǿ���ԡ�
(2)ȡ��������Һ����������CCl4������������ˮ������CCl4���Ϻ�ɫ��
(3)��ȡ��������Һ��μ���NaOH��Һ��ʹ��Һ��������Ϊ���ԣ���Ӧ�����о�����������
(4)ȡ����������(3)����Һ��Na2CO3��Һ���а�ɫ�������ɡ�
��������ʵ����ʵ����ȷ��������Һ�п϶����ڵ������� ��������ȷ���Ƿ���ڵ������� ��
���𰸡�
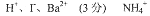
��������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ