��Ŀ����
 ʵ����������ͼװ�ý����к��ȵIJⶨ����ش��������⣺
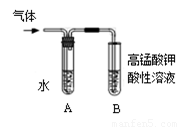
ʵ����������ͼװ�ý����к��ȵIJⶨ����ش��������⣺��1���ڲ�����ȷ��ǰ���£�����к��Ȳⶨȷ�ԵĹؼ���
����
����
��2����1���������к��Ȳⶨʵ�飬�¶ȼ���ʹ��
3
3
�Σ�ijͬѧΪ��ʡȥ��ϴ�¶ȼƵ��鷳������ʵ��ʱʹ����֧�¶ȼƷֱ������ͼ���¶ȣ����Ƿ�ͬ���ͬѧ�Ĺ۵㣬Ϊʲô��
��ͬ�⣬��Ϊ��ͬ�¶ȼ�����
��ͬ�⣬��Ϊ��ͬ�¶ȼ�����
��3��д��ϡ�����ϡ����������Һ��Ӧ��ʾ�к��ȵ��Ȼ�ѧ����ʽ���к���Ϊ57.3kJ/mol��
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
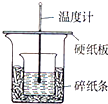
��4������NaOH��Һ����ȷ�����ǣ�������ѡ������
C
C
A���ز������������롡B���������������� C��һ��Ѹ�ٵ���
��5��ʹ������NaOH��Һ��Ͼ��ȵ���ȷ������E��������ѡ������
D
D
A�����¶ȼ�С�Ľ��� B���ҿ�ӲֽƬ�ò���������
C����������ձ� D���������¶ȼ��ϵĻ��β���������ؽ�����
��������1�����кͷ�Ӧ�У�����ȷ��������ɢʧ��
��2�������¶ȼ�Ҫ�ⷴӦǰ����Һ���¶ȣ��ⷴӦǰ����Һ���¶ȣ���Ϸ�Ӧ�������¶�һ��3�Σ����ݲ�ͬ�¶ȼƵ���ͬ��
��3�������к��ȵĸ�����������
��4������������������Ʒ�Ӧ��ӦѸ�پ��ң������ͷŴ����ȣ�һ���Կ��ٵ�����Լ�������ɢ������������
��5�����ݻ��β���������ʹ������NaOH��Һ��Ͼ��ȣ�
��2�������¶ȼ�Ҫ�ⷴӦǰ����Һ���¶ȣ��ⷴӦǰ����Һ���¶ȣ���Ϸ�Ӧ�������¶�һ��3�Σ����ݲ�ͬ�¶ȼƵ���ͬ��
��3�������к��ȵĸ�����������
��4������������������Ʒ�Ӧ��ӦѸ�پ��ң������ͷŴ����ȣ�һ���Կ��ٵ�����Լ�������ɢ������������
��5�����ݻ��β���������ʹ������NaOH��Һ��Ͼ��ȣ�
����⣺��1�����кͷ�Ӧ�У�����ȷ��������ɢʧ���ʴ�Ϊ�����£�
��2���¶ȼ�Ҫ�ⷴӦǰ����Һ���¶ȣ��ⷴӦǰ����Һ���¶ȣ���Ϸ�Ӧ�������¶�һ��3�Σ���ͬ�¶ȼƵ���ͬ���ʴ�Ϊ��3����ͬ�⣬��Ϊ��ͬ�¶ȼ����
��3��ϡ�����ϡ����������Һ��Ӧ��ʾ�к��ȵ��Ȼ�ѧ����ʽ��
H2SO4��l��+NaOH��l��=
Na2SO4��l��+H2O��l����H=-57.3KJ/mol���ʴ�Ϊ��
H2SO4��l��+NaOH��l��=
Na2SO4��l��+H2O��l����H=-57.3KJ/mol��
��4������������������Ʒ�Ӧ��ӦѸ�پ��ң������ͷŴ����ȣ�һ���Կ��ٵ�����Լ�������ɢ��������������ѡ��C��
��5���������¶ȼ��ϵĻ��β���������ؽ���ʹ������NaOH��Һ��Ͼ��ȣ���ѡ��D��
��2���¶ȼ�Ҫ�ⷴӦǰ����Һ���¶ȣ��ⷴӦǰ����Һ���¶ȣ���Ϸ�Ӧ�������¶�һ��3�Σ���ͬ�¶ȼƵ���ͬ���ʴ�Ϊ��3����ͬ�⣬��Ϊ��ͬ�¶ȼ����
��3��ϡ�����ϡ����������Һ��Ӧ��ʾ�к��ȵ��Ȼ�ѧ����ʽ��
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
��4������������������Ʒ�Ӧ��ӦѸ�پ��ң������ͷŴ����ȣ�һ���Կ��ٵ�����Լ�������ɢ��������������ѡ��C��
��5���������¶ȼ��ϵĻ��β���������ؽ���ʹ������NaOH��Һ��Ͼ��ȣ���ѡ��D��
���������⿼��ѧ���к��Ȳⶨ��ʵ�飬���¹�����ʵ����ص㣬���Ը�����ѧ�������ش��ѶȲ���

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
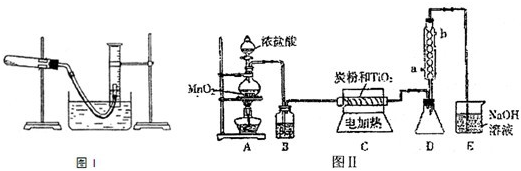
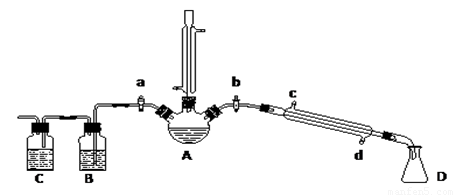
������ͼ1��ʾʵ��װ�ÿ��Բⶨһ��������1mol����������ͼ1������C��ΪҺ����ƿ��ƿ������110��130mL�̶��ߣ���һ��������þ�������ĺ�����������Aƿ����ȫ��Ӧ��������H2��Bƿ�е�Һ��ѹ��Һ����ƿ�У�����Һ����������ת����H2�������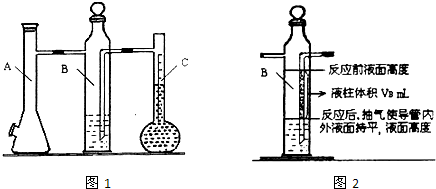
ʵ�鲽�裺
��1��װ��û�ѧ��Ӧ��������ⶨ�ǣ��������Լ�飮
��2����ɰƤ��ȥþ������������Ȼ���ȡ0.100g��0.110g��þ���������ݼ�¼�ڱ���
��3��ȡ��Aƿ���Ͽڵ���Ƥ������С�ձ�����20mLˮ���ٰ��ѳ�����þ���ӵ�Aƿ�ĵײ�������Ƥ���������Ͽڣ�
��4����ע������Aƿ���Ͽڴ�������ʹBƿ��������Һ���ƽ��
��5����ע������ȡ10mL 3mol/L���ᣬ����ͷ����Aƿ���Ͽ���Ƥ����������ע��Aƿ��ע���Ѹ�ٰγ���ͷ��
��6����þ����ȫ��Ӧ��ȡCƿ��Һ�������������ݼ�¼�ڱ���
��7����ע������Aƿ���Ͽڴ�������ʹBƿ�е�������Һ���ƽ����¼������������������ݼ�¼�ڱ���
�ظ������������еڶ���ʵ�飬����żȻ��
��������ʵ�鷽���ش��������⣺
ʵ�����¶ȣ�25�棬ѹǿ��101kPa����������1mol�������������ֵ��Ϊ24.5L
��1������װ�ü������Լ�飺��Aƿ���Ͽ����������� ����ȷ��װ�������Ժϸ�
��2��B����װҺ��һ���� ���ˮ����Ʒ�족����A�з�����Ӧ�����ӷ���ʽΪ ��
��3��ʵ���������£��¶ȣ�25�棨þԪ�ص����ԭ������Ϊ24.3��
���ϱ���X= ��
�ڼ���1mol�����������ʵ���ƽ��ֵ= L��
�ۼ���ʵ������ʵ��ֵ-����ֵ��/����ֵ��100%= ��
���������صĿ���ԭ�� ������ĸ����
A��þ���к��и������Ӧ������
B��û�г�ȥþ�����������þ
C��þ���к���������
D������ϡ�������
��4������ͬѧ��ʵ���в�õ�����ƫ�ߣ���¼����ʱ�ѻָ������£���Ϊ�ˣ�ij����ȤС���ͬѧ�Դ�ʵ�鷽������������������飺
��A��Bƿ�������к���ˮ�������ӵ����������������ˮ�������ü��������������
��Bƿ�е��ܣ�ͼ2����Ӱ����VB����Һ�������ڷ�Ӧ��Ϊ������ռ�ݣ����ü��������������
����Ϊ���ǵ������������ ������������ţ�������ޡ��������к���֮�����������������ݸ���������1mol�����������ѧ����ʽ����þԪ�ص����ԭ������Ϊ24.3��
1mol�������= L����д��ѧ����ʽ����

ʵ�鲽�裺
��1��װ��û�ѧ��Ӧ��������ⶨ�ǣ��������Լ�飮
��2����ɰƤ��ȥþ������������Ȼ���ȡ0.100g��0.110g��þ���������ݼ�¼�ڱ���
��3��ȡ��Aƿ���Ͽڵ���Ƥ������С�ձ�����20mLˮ���ٰ��ѳ�����þ���ӵ�Aƿ�ĵײ�������Ƥ���������Ͽڣ�
��4����ע������Aƿ���Ͽڴ�������ʹBƿ��������Һ���ƽ��
��5����ע������ȡ10mL 3mol/L���ᣬ����ͷ����Aƿ���Ͽ���Ƥ����������ע��Aƿ��ע���Ѹ�ٰγ���ͷ��
��6����þ����ȫ��Ӧ��ȡCƿ��Һ�������������ݼ�¼�ڱ���
��7����ע������Aƿ���Ͽڴ�������ʹBƿ�е�������Һ���ƽ����¼������������������ݼ�¼�ڱ���
�ظ������������еڶ���ʵ�飬����żȻ��
��������ʵ�鷽���ش��������⣺
ʵ�����¶ȣ�25�棬ѹǿ��101kPa����������1mol�������������ֵ��Ϊ24.5L
��1������װ�ü������Լ�飺��Aƿ���Ͽ�����������
��2��B����װҺ��һ����
��3��ʵ���������£��¶ȣ�25�棨þԪ�ص����ԭ������Ϊ24.3��
| ʵ����� | m��Mg��/g | �������/mL | Һ����ƿ��Һ�����/mL | ����������/mL | �������/mL | ����1mol�����/L |
| 1 | 0.100 | 10.0 | 110.0 | 6.5 | X | |
| 2 | 0.115 | 10.0 | 121.0 | 8.0 |
�ڼ���1mol�����������ʵ���ƽ��ֵ=
�ۼ���ʵ������ʵ��ֵ-����ֵ��/����ֵ��100%=
���������صĿ���ԭ��
A��þ���к��и������Ӧ������
B��û�г�ȥþ�����������þ
C��þ���к���������
D������ϡ�������
��4������ͬѧ��ʵ���в�õ�����ƫ�ߣ���¼����ʱ�ѻָ������£���Ϊ�ˣ�ij����ȤС���ͬѧ�Դ�ʵ�鷽������������������飺
��A��Bƿ�������к���ˮ�������ӵ����������������ˮ�������ü��������������
��Bƿ�е��ܣ�ͼ2����Ӱ����VB����Һ�������ڷ�Ӧ��Ϊ������ռ�ݣ����ü��������������
����Ϊ���ǵ������������
| ʵ����� | m��Mg�� g |
�������mL | Һ����ƿ��Һ�����mL | ����������mL | Bƿ��һ��Һ�����mL | ˮ������ٷֺ��� | ����1mol�����L |
| 1 | 0.100 | 10.0 | 110.0 | 6.5 | VB | a% |