��Ŀ����
19���������о������ʣ���HCl�����Cu�������ǡ���CO2����H2SO4����Ba��OH��2���塡 ���������Һ����ϡ���ᡡ������Al2��SO4��3��1�����ڵ���ʵ��Ǣ٢ݢޢ���ڷǵ���ʵ��Ǣۢܣ�
��2���ں͢෴Ӧ�Ļ�ѧ����ʽΪ��3Cu+8HNO3�T3Cu��NO3��2+2NO��+4H2O
������Ӧ������������3Cu��NO3��2������û��ȫ���μ�������ԭ��Ӧ���μ�������ԭ��Ӧ������ռ�������1��4�����ֵ����
��˫���ŷ�����������Ӧ��ֻ�������ӵ�ʧ�ķ������Ŀ��
3Cu+8HNO3�T3Cu��NO3��2+2NO��+4H2O
��3������������������Щ����֮��ɷ������ӷ�Ӧ��H++OH-�TH2O����д�������ӷ�Ӧ��Ӧ��һ����ѧ����ʽBa��OH��2+2HNO3=Ba��NO3��2+2H2O��
��4������ˮ�еĵ��뷽��ʽΪAl2��SO4��3=2Al3++3SO42-��
��5��3.42g ������ˮ���100mL��Һ��SO42- �����ʵ���Ũ��Ϊ0.3mol/L��
���� ��1����ˮ��Һ�������״̬���ܵ���Ļ������ǵ���ʣ������ᡢ��Ρ����ý����������ˮ��
��ˮ��Һ�������״̬�¶����ܵ���Ļ������Ƿǵ���ʣ�����һЩ�ǽ��������������������л�������ǡ��ƾ��ȣ���
��2��������ԭ��Ӧ������Ԫ�ػ��ϼ����ߵķ�Ӧ��Ϊ��ԭ������Ӧ����Ϊ����������������е�Ԫ�ػ��ϼ۱仯�ļ���μ�������ԭ��Ӧ������ռ�������������
����CuԪ�صĻ��ϼ۱仯��NԪ�صĻ��ϼ۱仯�����������ϼ����ߵ�Ԫ��ԭ��ʧȥ���ӣ����ϼ۽��͵�Ԫ�ص�ԭ�ӵõ����ӣ����ϼ�����ֵ=���ϼ۽���ֵ=ת�Ƶ�������
��3��H++OH-�TH2O�����Ա�ʾǿ����ǿ�Ӧ���ɿ������κ�ˮ��
��4��������Ϊǿ����ʣ�ˮ��Һ����ȫ���룻
��5������3.42g������������ʵ������������������뷽��ʽ������������ӵ����ʵ���������C=$\frac{n}{V}$������������ӵ����ʵ���Ũ�ȣ�
��� �⣺��1����HCl������ˮ��Һ���ܵ���Ļ�����ǵ���ʣ�
��Cu�ǵ��ʣ��Ȳ��ǵ����Ҳ���Ƿǵ���ʣ�
��������ˮ��Һ�������״̬�¶����ܵ���Ļ�����Ƿǵ���ʣ�
��CO2�������ܵ��룬���ڷǵ���ʣ�
��H2SO4����ˮ��Һ���ܵ���Ļ������ǵ���ʣ�
��Ba��OH��2��������״̬���ܹ�����Ļ�����ǵ���ʣ�
���������Һ�ǻ����Ȳ��ǵ����Ҳ���Ƿǵ���ʣ�
��ϡ�����ǻ����Ȳ��ǵ����Ҳ���Ƿǵ���ʣ�
������Al2��SO4��3��ˮ��Һ�������״̬���ܵ���Ļ�����ǵ���ʣ�
���ڵ���ʵ��� �٢ݢޢ���ڷǵ���ʵ��� �ۢܣ�
�ʴ�Ϊ���٢ݢޢ �ۢܣ�
��2��3Cu+8HNO3�T3Cu��NO3��2+2NO��+4H2O����Ӧ��ͭԪ�ػ��ϼ����ߣ�Ϊ��ԭ������Ӧ��������ͭΪ��������μӷ�Ӧ��������8mol��ֻ��2mol�����е�N���ϼ۽��ͣ������������μ�������ԭ��Ӧ������ռ�������1��4��
�ڷ�Ӧ3Cu+8HNO3=3Cu��NO3��2+2NO��+4H2O�У�CuԪ�صĻ��ϼ���0���ߵ�+2�ۣ�NԪ�صĻ��ϼ���+5����Ϊ+2�ۣ�ת�Ƶĵ���Ϊ6e-����˫���ŷ�������ӵ�ʧ�ķ������ĿΪ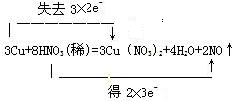 ��
��
�ʴ�Ϊ��Cu��NO3��2��1��4��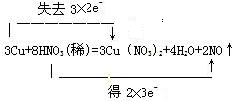 ��
��
��3��H++OH-�TH2O�����Ա�ʾ����������������Ӧ������ʽ��Ba��OH��2+2HNO3=Ba��NO3��2+2H2O��
�ʴ�Ϊ��Ba��OH��2+2HNO3=Ba��NO3��2+2H2O��
��4��������Ϊǿ����ʣ���ȫ���룬���뷽��ʽΪ��Al2��SO4��3=2Al3++3SO42-��
�ʴ�Ϊ��Al2��SO4��3=2Al3++3SO42-��
��5��3.42g �����������ʵ���n=$\frac{3.42g}{342g/mol}$=0.01mol���������������뷽��ʽ��Al2��SO4��3=2Al3++3SO42-����֪��������ӵ����ʵ���Ϊ0.03mol������������ӵ����ʵ���Ũ��C=$\frac{0.03mol}{0.1L}$=0.3mol/L��
�ʴ�Ϊ��0.3mol/L��
���� ���⿼���˵���ʡ��ǵ�����жϡ����뷽��ʽ�����ӷ���ʽ����д����ȷ����ʡ��ǵ���ʸ����ǽ���ؼ�����Ŀ�ѶȲ���

 �ö��Ե缫���ú��Һ�ķ�����H2�ķ�ӦΪC��s��+2H2O��l��=CO2��g��+2H2��g�����ֽ�һ������1mol/LH2SO4��Һ������ú�۳�ֻ�ϣ��Ƴɺ�̼��Ϊ0.02g/mL��0.12g/mL��ú��Һ��������ͼ��ʾװ���н��е�⣨���缫��Ϊ���Ե缫��������˵��������ǣ�������
�ö��Ե缫���ú��Һ�ķ�����H2�ķ�ӦΪC��s��+2H2O��l��=CO2��g��+2H2��g�����ֽ�һ������1mol/LH2SO4��Һ������ú�۳�ֻ�ϣ��Ƴɺ�̼��Ϊ0.02g/mL��0.12g/mL��ú��Һ��������ͼ��ʾװ���н��е�⣨���缫��Ϊ���Ե缫��������˵��������ǣ�������| A�� | A����������B��Ϊ���� | |
| B�� | A���ĵ缫��ӦʽΪC+2H2O-4e-=CO2��+4H+ | |
| C�� | B���ĵ缫��ӦʽΪ2H++2e-=H2�� | |
| D�� | ���һ��ʱ���ú��Һ��pH���� |
| A�� | HCl�ĵ���ʽ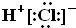 | B�� | CH4�����ģ�ͣ�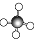 | ||
| C�� | S2-�Ľṹʾ��ͼΪ | D�� | ��ϩ�Ľṹ��ʽ�� |
| A�� | ������ԭ��Ӧ��ʵ���ǻ��ϼ۵����� | |
| B�� | ͬ��ͬѹ�£��κ�����ķ��Ӽ���뼸����� | |
| C�� | �����˶��ǽ���������������ɢϵ�ı������� | |
| D�� | SO2��ˮ��Һ�ܹ����磬����SO2�ǵ���� |
| A�� | ���ȷ�ұ�����۽��� | B�� | ʵ������NH4Cl��Ca��OH��2�Ʊ�NH3 | ||
| C�� | Na2O2����������ߵĹ����� | D�� | ��ҵ�ϵ������״̬Al2O3�Ʊ�Al |
| A�� | ����þ��Һ������������Һ��Ӧ��SO${\;}_{4}^{2-}$+Ba2+�TBaSO4�� | |
| B�� | ������NaHSO4��Ba��OH��2��Һ��Ӧ��Ba2++2OH-+2H++SO${\;}_{4}^{2-}$�TBaSO4��+2H2O | |
| C�� | ͭƬ������������Һ�У�Cu+Ag+�TCu2++Ag | |
| D�� | ����ʯ��ˮ�м������Ca��OH��2+2H+�TCa2++2H2O |
| A�� |  ������Һ | B�� | 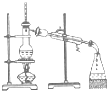 �����Ҵ���ˮ | ||
| C�� | 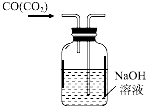 ��ȥCO�����е�CO2 | D�� |  ��ȥ�����еIJ����� |