��Ŀ����
���γ���������Ca2+��Mg2+��SO42-�Լ���ɳ�����ʣ�ʵ�����ᴿNaCl���������£���������Լ��Թ�����
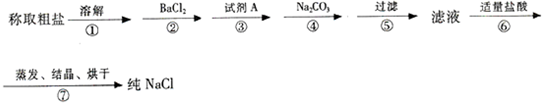
��1��������м�����Լ�A��
��2������ڡ��ۡ����õ����Լ�������������������˳����
��3��������У���ص����ӷ���ʽ
��4������������벽��Ե������������pH�ٹ��ˣ�����ʵ����������Ӱ����
��5��ʵ�����ᴿNaCl�Ĺ����У����ܽ⡢���ˡ�������������IJ����ж�Ҫ�ò�����������������ʱ��ʹ�õ�Ŀ����

��1��������м�����Լ�A��
NaOH
NaOH
���ѧʽ������ȥ��������Mg2+
Mg2+
���Լ�A�Թ�����Ŀ����ʹMg2+������ȫ
ʹMg2+������ȫ
����Ӧ�����ӷ���ʽMg2++2OH-�TMg��OH��2��
Mg2++2OH-�TMg��OH��2��
����2������ڡ��ۡ����õ����Լ�������������������˳����
BaCl2��Na2CO3��NaOH��NaOH��BaCl2��Na2CO3
BaCl2��Na2CO3��NaOH��NaOH��BaCl2��Na2CO3
����д��ѧʽ������3��������У���ص����ӷ���ʽ
Ca2++CO32-=CaCO3����Ba2++CO32-=BaCO3��
Ca2++CO32-=CaCO3����Ba2++CO32-=BaCO3��
����4������������벽��Ե������������pH�ٹ��ˣ�����ʵ����������Ӱ����
�ȼ�HCl��������CaCO3��BaCO3��Mg��OH��2�����ܽ⣬ʹNaCl�������ʣ��Ӷ�Ӱ���Ƶ�NaCl�Ĵ���
�ȼ�HCl��������CaCO3��BaCO3��Mg��OH��2�����ܽ⣬ʹNaCl�������ʣ��Ӷ�Ӱ���Ƶ�NaCl�Ĵ���
����5��ʵ�����ᴿNaCl�Ĺ����У����ܽ⡢���ˡ�������������IJ����ж�Ҫ�ò�����������������ʱ��ʹ�õ�Ŀ����
���裬��ֹ�ֲ�����Һ�λ���ɽ�
���裬��ֹ�ֲ�����Һ�λ���ɽ�
������������þ���ӻ������������Ӻ�̼����������ɳ����������ӻ���̼����������ɳ�������������ӻ��뱵�������ɳ���������������Գ�ȥ������̼���ƺ��������ƵȽ��з�����
��1�������ӿɳ����������ʣ��þ���Ӻ����ӣ�
��2��̼���ƿ��Խ��������Լ������ı����ӳ���������
��3�������ᴿʱ������̼���ƿ��Խ������ӺͶ���ı����ӳ�ȥ��д������̼��ơ�̼�ᱵ�����ӷ���ʽ��
��4��CaCO3��BaCO3��Mg��OH��2���������ܽ��������У�����һ�������ʣ�����Ч�����ã�
��5������ʱͨ���������Ľ������ã�ʹ��Һ�������Ⱦ��ȣ�
��1�������ӿɳ����������ʣ��þ���Ӻ����ӣ�
��2��̼���ƿ��Խ��������Լ������ı����ӳ���������
��3�������ᴿʱ������̼���ƿ��Խ������ӺͶ���ı����ӳ�ȥ��д������̼��ơ�̼�ᱵ�����ӷ���ʽ��
��4��CaCO3��BaCO3��Mg��OH��2���������ܽ��������У�����һ�������ʣ�����Ч�����ã�
��5������ʱͨ���������Ľ������ã�ʹ��Һ�������Ⱦ��ȣ�
����⣺��1�������ӿɳ����������AΪ�������ƣ��ɳ���þ���ӣ�Mg2++2OH-�TMg��OH��2�����ʴ�Ϊ��NaOH��Mg2+��ʹMg2+������ȫ��Mg2++2OH-�TMg��OH��2����
��2�������Լ�Ϊ�˳�ȥ�������ӣ�һ���ǹ����ģ�̼���ƿ��Խ��������Լ������ı����ӳ���������BaCl2��NaOH��Na2CO3�����˳���������������̼���ƣ����˺��ټ������Ʋ������������ɼ��ɣ������Լ�BaCl2��NaOH��Na2CO3�����˳������NaOH��BaCl2��Na2CO3������BaCl2��Na2CO3��NaOH�����ʴ�Ϊ��BaCl2��Na2CO3��NaOH��NaOH��BaCl2��Na2CO3��
��3������ܼ���̼������Һ����ȥ��Һ�еĸ����Ӽ������Ȼ��������ı��������ʣ���Ӧ�����ӷ���ʽΪ��Ca2++CO32-=CaCO3����Ba2++CO32-=BaCO3����
�ʴ�Ϊ��Ca2++CO32-=CaCO3����Ba2++CO32-=BaCO3����
��4�����������ܹ���̼�ᱵ��̼��ơ�������þ������Ӧ�����������pH�ٹ��ˣ���������CaCO3��BaCO3��Mg��OH��2�����ܽ⣬ʹNaCl�������ʣ��ʴ�Ϊ���ȼ�HCl��������CaCO3��BaCO3��Mg��OH��2�����ܽ⣬ʹNaCl�������ʣ��Ӷ�Ӱ���Ƶ�NaCl�Ĵ��ȣ�
��5��������ʱ��ʹ�ò��������н��裬�ܹ����Ⱦ��ȣ����Է�ֹ�ֲ�����Һ�λ���ɽ����ʴ�Ϊ�����裬��ֹ�ֲ�����Һ�λ���ɽ���
��2�������Լ�Ϊ�˳�ȥ�������ӣ�һ���ǹ����ģ�̼���ƿ��Խ��������Լ������ı����ӳ���������BaCl2��NaOH��Na2CO3�����˳���������������̼���ƣ����˺��ټ������Ʋ������������ɼ��ɣ������Լ�BaCl2��NaOH��Na2CO3�����˳������NaOH��BaCl2��Na2CO3������BaCl2��Na2CO3��NaOH�����ʴ�Ϊ��BaCl2��Na2CO3��NaOH��NaOH��BaCl2��Na2CO3��
��3������ܼ���̼������Һ����ȥ��Һ�еĸ����Ӽ������Ȼ��������ı��������ʣ���Ӧ�����ӷ���ʽΪ��Ca2++CO32-=CaCO3����Ba2++CO32-=BaCO3����
�ʴ�Ϊ��Ca2++CO32-=CaCO3����Ba2++CO32-=BaCO3����
��4�����������ܹ���̼�ᱵ��̼��ơ�������þ������Ӧ�����������pH�ٹ��ˣ���������CaCO3��BaCO3��Mg��OH��2�����ܽ⣬ʹNaCl�������ʣ��ʴ�Ϊ���ȼ�HCl��������CaCO3��BaCO3��Mg��OH��2�����ܽ⣬ʹNaCl�������ʣ��Ӷ�Ӱ���Ƶ�NaCl�Ĵ��ȣ�
��5��������ʱ��ʹ�ò��������н��裬�ܹ����Ⱦ��ȣ����Է�ֹ�ֲ�����Һ�λ���ɽ����ʴ�Ϊ�����裬��ֹ�ֲ�����Һ�λ���ɽ���
���������⿼���˴��ε��ᴿ�����ӷ���ʽ����д����Ŀ�ѶȲ���ע��̼���ƿ��Խ��������Լ������ı����ӳ���������

��ϰ��ϵ�д�
�����Ŀ