��Ŀ����
��1����֪KClO3+NH4NO2��KCl+NH4NO3ָ�����Ϸ�Ӧ�е���������______����������Ԫ����______ ��������ϼۣ���
��2����190g MgCl2����ˮ���Ƴ�1L��Һ��
�ٸ���Һ��MgCl2�����ʵ���Ũ��Ϊ______����Һ��Cl-�����ʵ���Ũ��Ϊ______��
������1mol?L-1��MgCl2��Һ500mL�������Һ�����Ϊ______��
�������500mL��Һ����ͨ��һ������HCl�������Һ��Cl-�����ʵ���Ũ��Ϊ3mol?L-1��������Һ������䣩������Һ��H+�����ʵ���Ũ��Ϊ______��ͨ��HCl������������״���£�Ϊ______��
�⣺
��1����KClO3�е���Ԫ�صĻ��ϼ���+5�۽��͵�-1�ۣ�����KClO3����������NH4NO2��������������ϵ�N��+3�����ߵ�+5�ۣ����������ʴ�Ϊ��KClO3��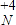
��2��
��MgCl2�����ʵ���Ϊn��MgCl2��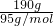 =2mol����MgCl2���ʵ���Ũ��ΪC��MgCl2��
=2mol����MgCl2���ʵ���Ũ��ΪC��MgCl2��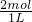 =2 mol/L����Һ��Cl-�����ʵ���Ũ��C��Cl-��=2C��MgCl2��=4mol/L���ʴ�Ϊ��2mol/L��4mol/L��
=2 mol/L����Һ��Cl-�����ʵ���Ũ��C��Cl-��=2C��MgCl2��=4mol/L���ʴ�Ϊ��2mol/L��4mol/L��
������Һϡ��ǰ�����ʵ����ʵ������䣬���������Һ�����ΪV=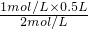 =0.25L���ʴ�Ϊ��0.25L��
=0.25L���ʴ�Ϊ��0.25L��
�������Cl-�����ʵ���Ũ��2 mol/L��ͨ��һ������HCl�������Һ��Cl-�����ʵ���Ũ��Ϊ3mol/L��������HCl��������ˮ�������Cl-�����ʵ���Ũ��Ϊ1mol/L��H+�����ʵ���Ũ��ҲΪ1mol/L��HCl�����ʵ���Ϊ��1mol/L��0.5L=0.5mol�����Ϊ0.5mol��22.4L/mol=11.2L���ʴ�Ϊ��1mol/L��11.2L��
������
��1�������������Ļ��ϼ۽��ͣ����ϼ����ߵ�Ԫ�ر�������
��2��
�ٸ���MgCl2���������MgCl2�����ʵ�����Ȼ�����C= ������ʵ���Ũ�ȣ�����MgCl2�����ʵ���Ũ���Լ�1��MgCl2�к���2�����������Cl-�����ʵ���Ũ�ȣ�����Һ������أ�
������ʵ���Ũ�ȣ�����MgCl2�����ʵ���Ũ���Լ�1��MgCl2�к���2�����������Cl-�����ʵ���Ũ�ȣ�����Һ������أ�
�ڸ�����Һϡ��ǰ�����ʵ����ʵ������䣻
�۸��ݢڿ�֪Cl-�����ʵ���Ũ��2 mol/L��ͨ��һ������HCl�������Һ��Cl-�����ʵ���Ũ��Ϊ3mol/L��������HCl��������ˮ�������Cl-�����ʵ���Ũ��Ϊ1mol/L��Ȼ�����HCl�����ʵ���������Լ�H+�����ʵ���Ũ�ȣ�
���������⿼�����ʵ��йؼ��㣬��Ŀ�ѶȲ�����ע��������ʵ�����������Ũ�ȵ��������ļ��㹫ʽ�����ã�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�