��Ŀ����
��18�֣���ҵβ���е�������ͨ�����ð������շ�����ԭ����NH3��NOx�ڴ��������·�Ӧ�����������ʡ�ijУ�С��ͬѧ��������װ�úͲ���ģ�ҵ�ϵ������� �Ĵ������̡�
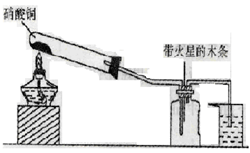
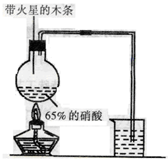
I��̽����ȡNH3�ķ���
��1��Bװ�õ����ƣ�______________
��2��������װ���У�H�ܿ��١������ȡ��װ������Ҫ���ӵķ�Ӧ�Լ�Ϊ_________ ��
��3��Ϊ̽�����õ�ʵ��Ч�����С��ͬѧ��������Cװ������ȡ�������ڿ���ʵ��������ͬ������£�����±���ʵ�����ݡ�
�����������ݣ�����Ϊ���ַ�����ȡ������Ч�����________������ţ����Ӹ÷���ѡ���ԭ�Ϸ�������Ч���õĿ���ԭ����________��__________��
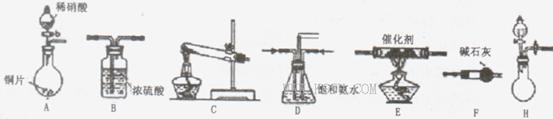
II.ģ��β������
�С��ͬѧѡ����������װ�ã�������˳�����ӳ�ģ��β������װ�ý���ʵ�顣
��1���������װ����ѡ������Ϊ�����Ľ��в��䣨��ѡװ�ò����ظ�����

��2��A�з�Ӧ�����ӷ���ʽΪ__________________________
��3��Dװ�õ������У�_____________��____________��_____________��
��4��Dװ���е�Һ�廹�ɻ��� ������ţ���
A��H2OB��CCl4C��ŨH2SO4D��CuSO4��Һ
��5����С��ͬѧ����Ƶ�ģ��β������װ���л�����һ�����Ե�ȱ����___________.

I��̽����ȡNH3�ķ���
��1��Bװ�õ����ƣ�______________
��2��������װ���У�H�ܿ��١������ȡ��װ������Ҫ���ӵķ�Ӧ�Լ�Ϊ_________ ��
��3��Ϊ̽�����õ�ʵ��Ч�����С��ͬѧ��������Cװ������ȡ�������ڿ���ʵ��������ͬ������£�����±���ʵ�����ݡ�
�����������ݣ�����Ϊ���ַ�����ȡ������Ч�����________������ţ����Ӹ÷���ѡ���ԭ�Ϸ�������Ч���õĿ���ԭ����________��__________��
| �Լ������� | �����Լ� | NH3�����mL�� | |
| a | 6.0gCa(OH)2���� | 5.4gNH4Cl | 1344 |
| b | 5.4g(NH4)2SO4 | 1364 | |
| c | 6.0gNaOH���� | 5.4gNH4Cl | 1568 |
| d | 5.4g(NH4)2SO4 | 1559 | |
| e | 6.0gCaO���� | 5.4gNH4Cl | 1753 |
| f | 5.4g(NH4)2SO4 | 1792 | |
II.ģ��β������
�С��ͬѧѡ����������װ�ã�������˳�����ӳ�ģ��β������װ�ý���ʵ�顣
��1���������װ����ѡ������Ϊ�����Ľ��в��䣨��ѡװ�ò����ظ�����

��2��A�з�Ӧ�����ӷ���ʽΪ__________________________
��3��Dװ�õ������У�_____________��____________��_____________��
��4��Dװ���е�Һ�廹�ɻ��� ������ţ���
A��H2OB��CCl4C��ŨH2SO4D��CuSO4��Һ
��5����С��ͬѧ����Ƶ�ģ��β������װ���л�����һ�����Ե�ȱ����___________.
��18�֣���1��ϴ��ƿ(1��)
��2����ʯ����Ũ��ˮ�����ռ���Ũ��ˮ������ѧʽ���֣�(2��)
��3��f (2��)����NH4��2��SO4�����Ȳ��ֽ⣬CaO������ˮ���ã������𰸾����֣�(2��)
��1��F E B(2��)
��2��3Cu+2NO3-+8H+=2NO��+4H2O +3Cu2+(2��)
��3��ʹ�����Ͼ��ȣ����������ٶȣ���ֹ�����ܽ⣨���������(3��)
��4��b(2��)
��5��δ��Ӧ��NO������������Ⱦ����(2��)
��2����ʯ����Ũ��ˮ�����ռ���Ũ��ˮ������ѧʽ���֣�(2��)
��3��f (2��)����NH4��2��SO4�����Ȳ��ֽ⣬CaO������ˮ���ã������𰸾����֣�(2��)
��1��F E B(2��)
��2��3Cu+2NO3-+8H+=2NO��+4H2O +3Cu2+(2��)
��3��ʹ�����Ͼ��ȣ����������ٶȣ���ֹ�����ܽ⣨���������(3��)
��4��b(2��)
��5��δ��Ӧ��NO������������Ⱦ����(2��)
�����������1��װ��B��������ϴ��ƿ��
��2���ܿ��١������ȡ���������Լ�ΪŨ��ˮ����ʯ�һ�Ũ��ˮ���������ƶ����ԣ�����H����Ҫ���ӵķ�Ӧ�Լ�Ϊ��ʯ����Ũ��ˮ�����ռ���Ũ��ˮ����
��3�����ݱ������ݣ���ͬ�����������Ӧ�����İ���������ͬ��f���������İ�����࣬�Ҽ���ʱ����鱗��ֽ⣬��ʯ�һ�������ˮ���ã����Ȼ�����ȷֽ�����������Ȼ��⣬�����ֽ�ϳ��Ȼ�泥��������ƻ�������������η�Ӧ������ˮ�϶࣬����f������ȡ������Ч����ã�
��1��װ��A���ɵ�NO��װ��C���ɵ�NH3ͨ��װ��Dʹ�����Ͼ��ȡ����������ٶȣ�װ��F�������壬��ͨ��װ��E��Ӧ���з�Ӧ����Ӧ�������ͨ��װ��B�������ռ����壬�ʴ�Ϊ��FEB��
��2��ϡ�������ǿ�����ԣ���ͭ����������ԭ��Ӧ��������ͭ��NO�ȣ���Ӧ�����ӷ���ʽΪ 3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
��3��Dװ��ʹ�����Ͼ��ȡ����������ٶȣ����⣬���������ڱ��Ͱ�ˮ���ɷ�ֹ������
��4����������������ͭ��Һ��ˮ�Լ������У��ɲ�������������Ϊ���Է��ӣ������ڷǼ����ܼ�������CCl4���汥�Ͱ�ˮ����ѡb��
��5��ȱ��β������װ�ã�������NO����Ⱦ������

��ϰ��ϵ�д�
�����Ŀ
 H+(aq)+OH-(aq)��H�� +57.3 kJ��mol��1
H+(aq)+OH-(aq)��H�� +57.3 kJ��mol��1