��Ŀ����
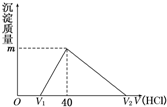
��2011?������0.80gCuSO4?5H2O��Ʒ������ˮ���̵��������ߣ���Ʒ�������¶ȱ仯�����ߣ���ͼ��ʾ��

��ش��������⣺
��1����ȷ��200��ʱ�������ʵĻ�ѧʽ
��2��ȡ270��������Ʒ����570�����յõ�����Ҫ�����Ǻ�ɫ��ĩ��һ�����������壬�÷�Ӧ�Ļ�ѧ����ʽΪ
��3������������������ˮ��Ӧ����һ�ֻ�����û������Ũ��Һ��Cu�ڼ���ʱ������Ӧ�Ļ�ѧ����ʽΪ
��4����0.10mol?L-1����ͭ��Һ�м�����������ϡ��Һ��ֽ��裬��dz��ɫ������ͭ�������ɣ�����Һ��pH=8ʱ��c��Cu2+��=

��ش��������⣺
��1����ȷ��200��ʱ�������ʵĻ�ѧʽ
CuSO4?H2O
CuSO4?H2O
��Ҫ��д���ƶϹ��̣�����2��ȡ270��������Ʒ����570�����յõ�����Ҫ�����Ǻ�ɫ��ĩ��һ�����������壬�÷�Ӧ�Ļ�ѧ����ʽΪ
CuSO4
CuO+SO3��
| ||
CuSO4
CuO+SO3��
���Ѹú�ɫ��ĩ�ܽ���ϡ�����У���Ũ������ȴ���о����������þ���Ļ�ѧʽΪ
| ||
CuSO4?5H2O
CuSO4?5H2O
������ڵ�����¶���102��
102��
����3������������������ˮ��Ӧ����һ�ֻ�����û������Ũ��Һ��Cu�ڼ���ʱ������Ӧ�Ļ�ѧ����ʽΪ
2H2SO4��Ũ��+Cu
CuSO4+SO2��+2H2O
| ||
2H2SO4��Ũ��+Cu
CuSO4+SO2��+2H2O
��
| ||
��4����0.10mol?L-1����ͭ��Һ�м�����������ϡ��Һ��ֽ��裬��dz��ɫ������ͭ�������ɣ�����Һ��pH=8ʱ��c��Cu2+��=
2.2��10-8
2.2��10-8
mol?L-1��Ksp[Cu��OH��2]=2.2��10-20��������0.1mol?L-1����ͭ��Һ��ͨ�����H2S���壬ʹCu2+��ȫ����ΪCuS����ʱ��Һ�е�H+Ũ����0.2
0.2
mol?L-1����������1����ͼ������֪��CuSO4?5H2O���ȵ�102��ʱ��ʼ��ˮ�ֽ⣬113��ʱ�ɵõ����ȶ���һ���м����258��ʱ�Ż�����ֽ⣮��200��ʱʧȥ��ˮ������Ϊ0.80g-0.57g=0.23g��������Ӧ�Ļ�ѧ����ʽ����ȷ����ʱ�������ʵĻ�ѧʽ��
��2���¶�Ϊ570�����յõ��ĺ�ɫ��ĩ��CuO��������������ΪSO3����Ӧ����ʽΪ��CuSO4
CuO+SO3����CuO��ϡ���ᷴӦ�IJ���������ͭ��ˮ������Ũ������ȴ�õ��ľ���Ϊ
CuSO4?5H2O������ͼ���������ڵ����102�棻
��3������Ũ�������ǿ�����Խ��з�����д���йصĻ�ѧ����ʽ��
��4�������ܶȻ��������м���c��Cu2+����������Һ�ĵ����Լ���H+Ũ�ȣ�
��2���¶�Ϊ570�����յõ��ĺ�ɫ��ĩ��CuO��������������ΪSO3����Ӧ����ʽΪ��CuSO4
| ||
CuSO4?5H2O������ͼ���������ڵ����102�棻
��3������Ũ�������ǿ�����Խ��з�����д���йصĻ�ѧ����ʽ��
��4�������ܶȻ��������м���c��Cu2+����������Һ�ĵ����Լ���H+Ũ�ȣ�
����⣺��1��CuSO4?5H2O���ȵ�102��ʱ��ʼ��ˮ�ֽ⣬113��ʱ�ɵõ����ȶ���һ���м����258��ʱ�Ż�����ֽ⣮��200��ʱʧȥ��ˮ������Ϊ0.80g-0.57g=0.23g��
���ݷ�Ӧ�Ļ�ѧ����ʽ��
CuSO4?5H2O
CuSO4?��5-n��H2O+nH2O
250 18n
0.80g 0.80g-0.57g=0.23g
=
�����n=4��
200��ʱ�ù������ʵĻ�ѧʽΪCuSO4?H2O���ʴ�ΪCuSO4?H2O��
��2���¶�Ϊ570�����յõ��ĺ�ɫ��ĩӦ��CuO��������������ΪSO3����Ӧ����ʽΪ��CuSO4
CuO+SO3����CuO��ϡ���ᷴӦ�IJ���������ͭ��ˮ��
����Ũ������ȴ�õ��ľ���ΪCuSO4?5H2O������ڵ����102�森�ʴ�Ϊ��CuSO4
CuO+SO3���� CuSO4?5H2O��102�棻
��3��SO3��ˮ��Ӧ�������ᣬŨ������ͭ���ȷ�Ӧ�Ļ�ѧ����ʽΪ��2H2SO4��Ũ��+Cu
CuSO4+SO2��+2H2O���ʴ�Ϊ��2H2SO4��Ũ��+Cu
CuSO4+SO2��+2H2O��
��4���������Cu��OH��2���ܶȻ�����ȷ��pH=8ʱ��c��OH-��=10-6mol/L��Ksp[Cu��OH��2]=2.2��10-20����c��Cu2+��=2.2��10-8mol?L-1����0.1mol?L-1����ͭ��Һ��ͨ��
����H2S���壬ʹCu2+��ȫ����ΪCuS����ʱ��Һ�е�����Ϊ���ᣬc��SO42-�����䣬Ϊ0.1mol?L-1���ɵ���غ��֪c��H+��Ϊ0.2mol?L-1���ʴ�Ϊ��2.2��10-8��0.2��
���ݷ�Ӧ�Ļ�ѧ����ʽ��
CuSO4?5H2O
| ||
250 18n
0.80g 0.80g-0.57g=0.23g
| 250 |
| 18n |
| 0.80g |
| 0.23g |
200��ʱ�ù������ʵĻ�ѧʽΪCuSO4?H2O���ʴ�ΪCuSO4?H2O��
��2���¶�Ϊ570�����յõ��ĺ�ɫ��ĩӦ��CuO��������������ΪSO3����Ӧ����ʽΪ��CuSO4
| ||
����Ũ������ȴ�õ��ľ���ΪCuSO4?5H2O������ڵ����102�森�ʴ�Ϊ��CuSO4
| ||
��3��SO3��ˮ��Ӧ�������ᣬŨ������ͭ���ȷ�Ӧ�Ļ�ѧ����ʽΪ��2H2SO4��Ũ��+Cu
| ||
| ||
��4���������Cu��OH��2���ܶȻ�����ȷ��pH=8ʱ��c��OH-��=10-6mol/L��Ksp[Cu��OH��2]=2.2��10-20����c��Cu2+��=2.2��10-8mol?L-1����0.1mol?L-1����ͭ��Һ��ͨ��
����H2S���壬ʹCu2+��ȫ����ΪCuS����ʱ��Һ�е�����Ϊ���ᣬc��SO42-�����䣬Ϊ0.1mol?L-1���ɵ���غ��֪c��H+��Ϊ0.2mol?L-1���ʴ�Ϊ��2.2��10-8��0.2��
���������⿼������ͭ�ᾧˮ�����IJⶨ���ܶȻ������ļ����Լ����ʵ���Ũ�ȵ��йؼ��㣬��Ŀ��Ϊ�ۺϣ�����ͼ����Ϣ����ɱ���Ŀ�Ĺؼ���

��ϰ��ϵ�д�
 Сѧ���AB��ϵ�д�
Сѧ���AB��ϵ�д�
�����Ŀ
 ��2011?������ģ����һ�����������Ͻ�����ˮ�У��Ͻ�ȫ���ܽ⣬�õ�20mL OH-Ũ��Ϊ1mol/L����Һ��Ȼ����1mol/L������ζ���������������������������ϵ��ͼ��ʾ��������ѡ����ȷ���ǣ�������
��2011?������ģ����һ�����������Ͻ�����ˮ�У��Ͻ�ȫ���ܽ⣬�õ�20mL OH-Ũ��Ϊ1mol/L����Һ��Ȼ����1mol/L������ζ���������������������������ϵ��ͼ��ʾ��������ѡ����ȷ���ǣ�������