��Ŀ����
�������ӷ���ʽ��д��ȷ����
| A��NaHSO4��Һ��Ba(OH)2��Һ��Ӧ�����ԣ�H++SO42��+Ba2++OH�� = BaSO4��+H2O |
| B������������KIO3��Һ��KI��Һ������Ӧ����I2�� IO3����5I����3H2O��3I2��6OH�� |
| C��NH4HCO3��Һ������ŨNaOH��Һ��Ӧ�� NH4+ +HCO3�� + 2OH-= NH3�� +2H2O+CO32- |
| D��������ˮ�ķ�Ӧ�� C12+H2O=2H++C1��+ClO�� |
C
A����ȷ��������ʱ������ʽΪ2NaHSO4��Ba(OH)2=BaSO4��2H2O��Na2SO4�����������²���������OH����Ӧ����ˮ��B����ȷ����������������ʣ��û�ѧʽ��ʾ��D�Dz���ȷ��������ȷ�Ĵ���C��

��ϰ��ϵ�д�
�����Ŀ
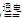 2OH����H2����Cl2��
2OH����H2����Cl2��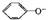 + CO2 + H2O �� 2
+ CO2 + H2O �� 2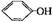 + CO32-
+ CO32-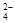 =BaSO4��
=BaSO4��